Vaccines, COVID-19 and Vaccine Hesitancy (Anti-vaxxers)
The origins of vaccinations go back hundreds of years ago. In Chinese, European and African societies, smallpox scabs/matter from pustules were introduced into the nasal passages, or through scratches or small incisions in the skin, to help protect against smallpox.1Rusnock AA. Historical context and the roots of Jenner's discovery. Hum Vaccin Immunother 2016;12(8):2025–8. doi: 10.1080/21645515.2016.1158369. It was also observed that cow maids acquired immunity to smallpox through exposure to cowpox, a related but less pathogenic virus.2Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111. Since then, mass immunization has dramatically altered the landscape for the transmission of many diseases, their morbidity and mortality.3Centers for Disease Control and Prevention. Benefits from Immunization During the Vaccines for Children Program Era — United States, 1994–2013. MMWR April 25, 2014;63(16):352-5.,4Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bulletin of the World Health Organization 2008;86(2):81-160. Available at: https://www.who.int/bulletin/volumes/86/2/07-040089/en/. The current COVID-19 pandemic necessitates the urgent development and delivery of an effective vaccine against SARS-CoV-2. Challenges include de novo research, minimizing the time required, the global scale of immunization and the potential impact of vaccine hesitancy on the achievement of herd immunity.
Historical Vaccine Developments
The first formal vaccine was developed in Europe in 1796, using cowpox to inoculate individuals for protection against smallpox.1Rusnock AA. Historical context and the roots of Jenner's discovery. Hum Vaccin Immunother 2016;12(8):2025–8. doi: 10.1080/21645515.2016.1158369.,5McCullers JA. Evolution, Benefits, and Shortcomings of Vaccine Management. J Manag Care Pharm 2007;13(7 Suppl B):S2-6. doi: 10.18553/jmcp.2007.13.s7-b.2a. A wide range of other live attenuated vaccines later became available, including for measles, mumps, rubella, varicella, polio and typhoid.2Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111. Options for live attenuation include using an animal pathogen to confer immunity (such as cowpox instead of smallpox), exposing a virus to oxygen or heat, or cultivation to develop an attenuated strain or RNA from two viruses cultured together.2Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111. Inactivated (killed) vaccines were first introduced in the 1890s for typhoid, cholera and plague.2Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111. Vaccines containing antibodies have been developed to promote destruction of the polysaccharide capsule around pathogens, e.g., Hemophilus influenzae type b, as well as vaccines consisting of fully or partially purified proteins that act as antigens to promote an immune response, e.g., an influenza vaccine.2Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111.,6Cate TR, Couch RB, Kasel JA, Six HR. Clinical trials of monovalent influenza A/New Jersey/76 virus vaccines in adults: Reactogenicity, antibody response, and antibody persistence. J Infect Dis. 1977;136(Suppl):S450–S455. Genetic engineering also enables vaccine development, such as through the removal of specific DNA to change a pathogen’s properties, for instance to reduce virulence, or placement of DNA into a less pathogenic microorganism that acts as a vector for the vaccine.2Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111.,5McCullers JA. Evolution, Benefits, and Shortcomings of Vaccine Management. J Manag Care Pharm 2007;13(7 Suppl B):S2-6. doi: 10.18553/jmcp.2007.13.s7-b.2a. (Table 1)
| Table 1. Vaccine technologies |
|---|
| Live attenuated vaccines |
| Inactivated (killed) vaccines |
| Antibodies to polysaccharide capsules |
| Purified proteins as antigens |
| Genetic engineering |
Steps in Vaccine Research and Development
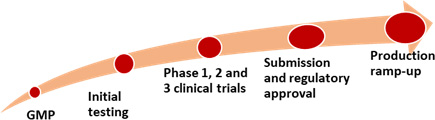
Researching and delivering a vaccine requires development of good manufacturing practices, initial testing for efficacy, safety and toxicity, and clinical trials.7Amanat F, Krammer F. Perspective. SARS-CoV-2 Vaccines: Status Report. Immunity 2020;52:583-589. Available at: https://doi.org/10.1016/j.immuni.2020.03.007. Phase 1 clinical trials involve a small number of individuals and assess a potential vaccine’s safety, while Phase 2 informs the vaccine’s dose and formulation. Phase 3 clinical trials involve a large number of subjects to evaluate the vaccine’s safety and efficacy. If successful results are achieved, submission is then made to regulatory bodies for approval which in the United States is the Food & Drug Administration. Ramping up production of the approved vaccine then takes place. The whole process typically takes several years.
Developing a Vaccine for COVID-19
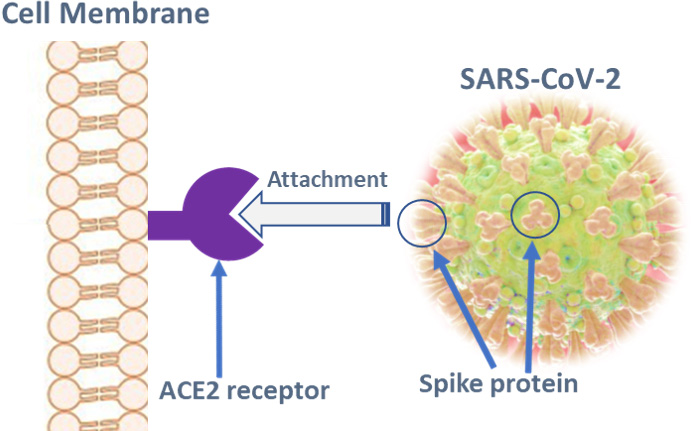
An effective vaccine against SARS-CoV-2, the causal agent for COVID-19, is critical in ending the current pandemic. SARS-CoV-2-related vaccine research has been undertaken with rapidity and with support from companies, educational and research institutions, organizations and governments.8Collins FS, Stoffels P. Viewpoint. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). An Unprecedented Partnership for Unprecedented Times. J Am Med Assoc May 18, 2020;E1-E3. [online]. Available at: https://jamanetwork.com. Approaches being researched include live attenuated, inactivated and genetically engineered vaccines, a variety of vehicles and vectors, and the use of virus-like particles.7Amanat F, Krammer F. Perspective. SARS-CoV-2 Vaccines: Status Report. Immunity 2020;52:583-589. Available at: https://doi.org/10.1016/j.immuni.2020.03.007.,9Chen W-H, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 Vaccine Pipeline: an Overview. Current Tropical Medicine Reports. Available at: https://doi.org/10.1007/s40475-020-00201-6.,10World Health Organization. DRAFT landscape of COVID-19 candidate vaccines – 9 June 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. As of June 9, 2020, 126 vaccine candidates were reported to be under pre-clinical research and 10 had reached clinical trial phases.10World Health Organization. DRAFT landscape of COVID-19 candidate vaccines – 9 June 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Many of the candidates target the viral spike (S) protein that attaches to and fuses with the ACE2 receptors of host cells. (Figure 2) This includes RNA vaccines, DNA vaccines, viral vector-based vaccines, virus-like nanoparticles and recombinant protein vaccines.7Amanat F, Krammer F. Perspective. SARS-CoV-2 Vaccines: Status Report. Immunity 2020;52:583-589. Available at: https://doi.org/10.1016/j.immuni.2020.03.007.,9Chen W-H, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 Vaccine Pipeline: an Overview. Current Tropical Medicine Reports. Available at: https://doi.org/10.1007/s40475-020-00201-6.,10World Health Organization. DRAFT landscape of COVID-19 candidate vaccines – 9 June 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.,11Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014;32(26):3169–74. https://doi.org/10.1016/j.vaccine.2014.04.016.
Among the 10 candidates already in clinical trials, 2 use a non-replicating viral vector and 1 an RNA platform. The non-replicating viral vector platform being researched by the University of Oxford/AstraZeneca is currently enrolling individuals in Phase 2b/3 of clinical trials. This platform has previously been used for 7 other diseases, including Middle East Respiratory Syndrome and influenza. The other two platforms include one by CanSino Biological Inc./Beijing Institute of Biotechnology and an mRNA candidate being researched by Moderna/National Institute of Allergy and Infectious Diseases.10World Health Organization. DRAFT landscape of COVID-19 candidate vaccines – 9 June 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. The latter is recruiting 30,000 adults for a Phase 3 randomized, controlled, multi-site international clinical trial due to begin shortly.12Abbasi J. Medical News & Analysis. Anthony Fauci,MD, on COVID-19 Vaccines, Schools, and Larry Kramer. J Am Med Assoc June 8, 2020;E1. Available at: https://jamanetwork.com. Four other candidates utilize inactivated vaccines and the remaining three already in clinical trials utilize RNA, DNA and protein subunit nanoparticle platforms.10World Health Organization. DRAFT landscape of COVID-19 candidate vaccines – 9 June 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
Current challenges
Current challenges include delivering a vaccine that is universally safe and effective, determining the duration of conferred immunity, and global immunization once a vaccine is proven safe and effective, and approved for use. Condensing the time required to achieve these goals is a further challenge. To address this, mechanisms have been developed to ramp up production and stockpile the mRNA vaccine before the final results of clinical trials and regulatory approval.12Abbasi J. Medical News & Analysis. Anthony Fauci,MD, on COVID-19 Vaccines, Schools, and Larry Kramer. J Am Med Assoc June 8, 2020;E1. Available at: https://jamanetwork.com. At the moment, it appears that it may be possible to ascertain the safety and efficacy of a vaccine before the end of this year and in tandem to ramp up production. This would enable the availability of around 100 million doses of the targeted vaccine by year end, and by early 2021 around double that number, should the vaccine prove to be safe and effective.12Abbasi J. Medical News & Analysis. Anthony Fauci,MD, on COVID-19 Vaccines, Schools, and Larry Kramer. J Am Med Assoc June 8, 2020;E1. Available at: https://jamanetwork.com. While an effective and safe vaccine is eagerly awaited, vaccine hesitancy is a further concern as a potential barrier to immunization at the population level.13Murphy J. What happens if some Americans refuse the COVID-19 vaccine? MD Linx, May 12, 2020. Available at: https://www.mdlinx.com/article/what-happens-if-some-americans-refuse-the-covid-19-vaccine/5Yqy3Q84Wt27BQktRnhGnD?utm_source=alert&utm_medium=email&utm_campaign=ajm_49890.
Vaccine hesitancy/anti-vaxxers
Vaccine hesitancy includes refusal to vaccinate, delays in vaccination or selective vaccination by individuals for themselves and their children.14Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. doi: 10.1001/jama.2016.1353.,15Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine 2014;32(19):2150-9. doi:10.1016/j.vaccine.2014.01.081. These individuals are often referred to as ‘anti-vaxxers.’ In a global survey conducted in 2014-2016, the most common reasons given for vaccine hesitancy were safety concerns/fear of side effects, lack of knowledge and awareness, and specific group attributes such as traditional beliefs or religion.16Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data-2015-2017. Vaccine 2018;36(26):3861‐7. doi:10.1016/j.vaccine.2018.03.063 Distrust and freedom of choice are also given as reasons, and some individuals state that compulsory vaccinations violate their civil liberties.17Diekema DS. Personal Belief Exemptions From School Vaccination Requirements. Annu Rev Public Health 2014.35:275-92. (Table 2) Disinformation, including on social media, websites, and through organized antivaccine lobbies, fosters vaccine hesitancy.18Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3.
| Table 2. Reasons given for vaccine hesitancy |
|---|
| Safety concerns/fear of side effects |
| Lack of knowledge and awareness |
| Traditional beliefs |
| Religious beliefs |
| Distrust and freedom of choice |
| Belief that compulsory vaccines violate civil liberties |
Misinformation has included an article published in 1998 suggesting a link between MMR vaccination and autism in 12 children that was later discredited and retracted, damages public health efforts and results in outbreaks of preventable transmissible diseases.18Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3.,19RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998;351[9103]:637–41.,20Eggertson L. Lancet retracts 12-year-old article linking autism to MMR vaccines. CMAJ 2010;182(4):E199‐E200. doi:10.1503/cmaj.109-3179. In the aftermath of the article, ‘tens of thousands’ of parents declined MMR vaccination for their children.20Eggertson L. Lancet retracts 12-year-old article linking autism to MMR vaccines. CMAJ 2010;182(4):E199‐E200. doi:10.1503/cmaj.109-3179. Yet, there is a substantial evidence base supporting the safety and efficacy of MMR vaccination, most recently evaluated in a Cochrane review of 51 and 87 studies, respectively, assessing the efficacy and safety of MMR vaccination in children under 15 years-of-age.21Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for Measles, Mumps, Rubella, and Varicella in Children. Cochrane Database Syst Rev 2020;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4. There is no evidence of an association between MMR immunization and autism, encephalitis, asthma, type 1 diabetes, dermatitis/eczema, hay fever, leukemia, cognitive delay, multiple sclerosis or bacterial/viral infections.21Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for Measles, Mumps, Rubella, and Varicella in Children. Cochrane Database Syst Rev 2020;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4.,22Swedish Council on Health Technology Assessment. Vaccines to Children: Protective Effect and Adverse Events: A Systematic Review [Internet]. 2009;SBU Yellow Report No. 191.
Impact of vaccine hesitancy and measles outbreaks
Prior to the availability of a vaccine in 1963, measles epidemics resulted in 2.6 million deaths globally each year, largely in young children.23World Health Organization. Key facts. Measles resurgence. Available at: https://www.who.int/news-room/fact-sheets/detail/measles. Subsequently, the number of deaths declined as global vaccination rates increased and by 2018, there were 142,000 deaths.23World Health Organization. Key facts. Measles resurgence. Available at: https://www.who.int/news-room/fact-sheets/detail/measles. In the United States, measles was stated to be eliminated in 2000.18Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3. However, measles has resurged in some countries, including the United States and Canada.18Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3.,24MacDonald NE, Dubé E. A New Resource to Summarize Evidence on Immunization From the Canadian Vaccination Evidence Resource and Exchange Centre (CANVax). Can Commun Dis Rep 2020;46(1):16-19. doi: 10.14745/ccdr.v46i01a03. Parents can request exemptions from mandated vaccinations for their children in most US states on philosophical and religious grounds. In one review from 2016, 18 studies were found with 1416 measles case. Seventy percent of non-vaccinated individuals had received philosophical or religious exemptions.14Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. doi: 10.1001/jama.2016.1353. Since then, measles outbreaks have occurred in the United States each year.25US Centers for Disease Control and Prevention. Measles: cases and outbreaks. Available at: https://www.cdc.gov/measles/cases-outbreaks.html.,26Sundaram ME, Guterman LB, Omer SB, Omer SB. The True Cost of Measles Outbreaks During the Postelimination Era. J Am Med Assoc. 2019;321(12):1155-6. doi: 10.1001/jama.2019.1506 Between December 30, 2018 and the end of 2019, 1282 measles cases were reported, the majority of cases again in unvaccinated individuals.25US Centers for Disease Control and Prevention. Measles: cases and outbreaks. Available at: https://www.cdc.gov/measles/cases-outbreaks.html.
Exemptions and Herd Immunity
Medical exemptions are given for individuals for whom a vaccine is contraindicated, including individuals with inherited immunodeficiencies. However, provided enough of the population is vaccinated, herd immunity is still achieved, which means that unvaccinated individuals are protected from exposure as they are surrounded by individuals who were vaccinated. Lower vaccination rates thus reduce community protection against disease.24MacDonald NE, Dubé E. A New Resource to Summarize Evidence on Immunization From the Canadian Vaccination Evidence Resource and Exchange Centre (CANVax). Can Commun Dis Rep 2020;46(1):16-19. doi: 10.14745/ccdr.v46i01a03. While it may be understandable that some individuals want the right to exempt themselves and their children from vaccination for reasons other than medical necessity, this is not without consequences. Other members of society, including the vulnerable, deserve to be protected. Prior to the current pandemic, we had not been exposed to SARS-CoV-2 and do not have pre-existing acquired immunity. Herd immunity through immunization could be achieved if most of the population has been vaccinated against SARS-CoV-2 once a vaccine is available. Unfortunately, recent research suggests that vaccine hesitancy may be an issue.27The conversation. A majority of vaccine skeptics plan to refuse a COVID-19 vaccine, a study suggests, and that could be a big problem, May 4, 2020. Available at: https://www.niaid.nih.gov/diseases-conditions/covid-19-clinical-research.
Conclusions
Immunization against many diseases has been a public health success over several decades.5McCullers JA. Evolution, Benefits, and Shortcomings of Vaccine Management. J Manag Care Pharm 2007;13(7 Suppl B):S2-6. doi: 10.18553/jmcp.2007.13.s7-b.2a. In addition to disease morbidity and mortality, other burdens associated with lack of/inadequate vaccination against transmissible diseases include the direct cost of care, potential need for quarantining, contact tracing, and weakening of healthcare systems.26Sundaram ME, Guterman LB, Omer SB, Omer SB. The True Cost of Measles Outbreaks During the Postelimination Era. J Am Med Assoc. 2019;321(12):1155-6. doi: 10.1001/jama.2019.1506 At the current time, an effective and safe vaccine is desperately needed to combat COVID-19 and its consequences, and adequate levels of vaccination will be essential to obtain herd immunity. It is to be hoped that messaging can be developed that addresses misinformation, and policies developed that ensure adequate vaccination at the community level. First and foremost, a safe and effective vaccine is needed.
References
- 1.Rusnock AA. Historical context and the roots of Jenner's discovery. Hum Vaccin Immunother 2016;12(8):2025–8. doi: 10.1080/21645515.2016.1158369.
- 2.Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014;111(34):12283-7. doi: 10.1073/pnas.1400472111.
- 3.Centers for Disease Control and Prevention. Benefits from Immunization During the Vaccines for Children Program Era — United States, 1994–2013. MMWR April 25, 2014;63(16):352-5.
- 4.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bulletin of the World Health Organization 2008;86(2):81-160. Available at: https://www.who.int/bulletin/volumes/86/2/07-040089/en/.
- 5.McCullers JA. Evolution, Benefits, and Shortcomings of Vaccine Management. J Manag Care Pharm 2007;13(7 Suppl B):S2-6. doi: 10.18553/jmcp.2007.13.s7-b.2a.
- 6.Cate TR, Couch RB, Kasel JA, Six HR. Clinical trials of monovalent influenza A/New Jersey/76 virus vaccines in adults: Reactogenicity, antibody response, and antibody persistence. J Infect Dis. 1977;136(Suppl):S450–S455.
- 7.Amanat F, Krammer F. Perspective. SARS-CoV-2 Vaccines: Status Report. Immunity 2020;52:583-589. Available at: https://doi.org/10.1016/j.immuni.2020.03.007.
- 8.Collins FS, Stoffels P. Viewpoint. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). An Unprecedented Partnership for Unprecedented Times. J Am Med Assoc May 18, 2020;E1-E3. [online]. Available at: https://jamanetwork.com.
- 9.Chen W-H, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 Vaccine Pipeline: an Overview. Current Tropical Medicine Reports. Available at: https://doi.org/10.1007/s40475-020-00201-6.
- 10.World Health Organization. DRAFT landscape of COVID-19 candidate vaccines – 9 June 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 11.Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014;32(26):3169–74. https://doi.org/10.1016/j.vaccine.2014.04.016.
- 12.Abbasi J. Medical News & Analysis. Anthony Fauci,MD, on COVID-19 Vaccines, Schools, and Larry Kramer. J Am Med Assoc June 8, 2020;E1. Available at: https://jamanetwork.com.
- 13.Murphy J. What happens if some Americans refuse the COVID-19 vaccine? MD Linx, May 12, 2020. Available at: https://www.mdlinx.com/article/what-happens-if-some-americans-refuse-the-covid-19-vaccine/5Yqy3Q84Wt27BQktRnhGnD?utm_source=alert&utm_medium=email&utm_campaign=ajm_49890.
- 14.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. doi: 10.1001/jama.2016.1353.
- 15.Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine 2014;32(19):2150-9. doi:10.1016/j.vaccine.2014.01.081.
- 16.Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data-2015-2017. Vaccine 2018;36(26):3861‐7. doi:10.1016/j.vaccine.2018.03.063
- 17.Diekema DS. Personal Belief Exemptions From School Vaccination Requirements. Annu Rev Public Health 2014.35:275-92.
- 18.Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3.
- 19.RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998;351[9103]:637–41.
- 20.Eggertson L. Lancet retracts 12-year-old article linking autism to MMR vaccines. CMAJ 2010;182(4):E199‐E200. doi:10.1503/cmaj.109-3179.
- 21.Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for Measles, Mumps, Rubella, and Varicella in Children. Cochrane Database Syst Rev 2020;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4.
- 22.Swedish Council on Health Technology Assessment. Vaccines to Children: Protective Effect and Adverse Events: A Systematic Review [Internet]. 2009;SBU Yellow Report No. 191.
- 23.World Health Organization. Key facts. Measles resurgence. Available at: https://www.who.int/news-room/fact-sheets/detail/measles.
- 24.MacDonald NE, Dubé E. A New Resource to Summarize Evidence on Immunization From the Canadian Vaccination Evidence Resource and Exchange Centre (CANVax). Can Commun Dis Rep 2020;46(1):16-19. doi: 10.14745/ccdr.v46i01a03.
- 25.US Centers for Disease Control and Prevention. Measles: cases and outbreaks. Available at: https://www.cdc.gov/measles/cases-outbreaks.html.
- 26.Sundaram ME, Guterman LB, Omer SB, Omer SB. The True Cost of Measles Outbreaks During the Postelimination Era. J Am Med Assoc. 2019;321(12):1155-6. doi: 10.1001/jama.2019.1506
- 27.The conversation. A majority of vaccine skeptics plan to refuse a COVID-19 vaccine, a study suggests, and that could be a big problem, May 4, 2020. Available at: https://www.niaid.nih.gov/diseases-conditions/covid-19-clinical-research.