Artificial Intelligence Applied to Dental Medicine
Artificial intelligence (AI) refers to development of machines that function and perform tasks analogous to those performed by humans. The field of AI began after the Second World War and has seen periods of great interest as well as disinterest and skepticism1Horgan j. Will artificial intelligence ever live up to its hype? Scientific american. Retrieved from: Https://www.Scientificamerican.Com/article/will-artificial-intelligence-ever-live-up-to-its-hype/ on december 6, 2020.. Recently, AI has attracted new attention, in large part due to advances in computer technology. Other important terms used in this context are “machine learning” (ML) which refers to an aspect of AI that uses algorithms to identify patterns in data sets and thereby predict such patterns in other data, and “deep learning” (DL), which is an aspect of ML that mimics the functions of the human brain, with its neural networks. What is implied here is that there are multiple levels within the system, and hence the “deep” descriptor2Schwendicke F, Samek W, Krois J. Artificial intelligence in dentistry: Chances and challenges. J Dent Res. 2020;99(7):769-74..
Interest in the application of AI in many aspects of society reflects the computing power, availability of large databases from which information can be mined, as well as the potential for removing human error from decision-making situations. We see evidence of AI in our daily lives. When you make a purchase on-line, and then receive follow-up messages offering similar products for sale, AI is at work.
The application of AI to healthcare is one obvious area of study and has experienced recent growth. This has occurred in several clinical fields. As examples in medicine, consider ophthalmology and diagnostic radiology.
Ophthalmology
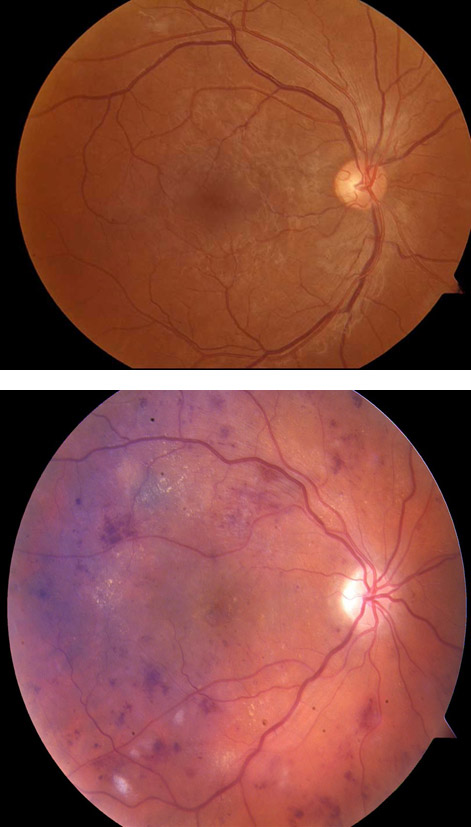
A recent review highlighted the application of AI in the identification of several eye disorders, including diabetic retinopathy, glaucoma, and age-related macular degeneration3Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-75.. Diabetic retinopathy (DR) is a clinical complication of diabetes, and it has been estimated that approximately one-third of patients with diabetes will develop this disorder. DR is identified using a variety of techniques that visualize the retina at the back of the eye (Figure 1). Screening is recognized as an important tool for early detection of DR, and referral for treatment to prevent blindness. The performance of AI/DL systems to detect DR has been shown to be was very good. Sensitivity (the percent of positive cases that are identified) ranged from 87% to 100%, and specificity (the percentage of negative cases that are identified) ranged from 73% to 98%. These results were seen with evaluation of existing data sets where the outcome is known. More recently, real-world clinical program assessments yielded comparably impressive results. Other studies have shown similar outcomes when AI/DL systems were used for identification of glaucoma and age-related macular degeneration.
The potential benefit of these systems for identification of DR is obvious. These techniques can be used as cost-saving approaches to screen patients with diabetes in resource poor countries and remote areas where an ophthalmologist is not available. These techniques could be linked to telemedicine for diagnosis and initiation of treatment.
However, challenges to implementation exist. These include the need to determine accuracy in different populations, standardization of image capture, application in other common (cataract) and rare (tumors) conditions, physician acceptance, medical-legal issues, and re-imbursement.
Diagnostic Radiology
The use of AI has been extensively examined in relation to medical imaging. Research has shown that AI systems are extremely effective at identification of the complex patterns that characterize many medical images, and can provide quantitative analysis of these patterns, with the intent of identifying abnormalities, primarily associated with disease4Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18(8):500-10..
A good example of the application of AI to diagnostic radiology is the chest x-ray. Since this image is commonly used throughout the world, existing data sets are available for evaluation. A review of the agreement between DL systems and experienced radiologists indicated close agreement, but that radiologists in different institutions may not agree as closely5Yasaka K, Abe O. Deep learning and artificial intelligence in radiology: Current applications and future directions. PLoS Med. 2018;15(11):e1002707.. Another study examined agreement as to identification of COVID-19 pneumonia. COVID-19 infection was verified by the reverse transcription polymerase chain reaction (223 positive, 231 negative). The AI system correctly identified COVID-19 pneumonia on radiographs with approximately 80% accuracy. This degree of accuracy was comparable to that achieved by independent radiologists who read the films6Murphy K, Smits H, Knoops AJG, Korst M, Samson T, Scholten ET, et al. Covid-19 on chest radiographs: A multireader evaluation of an artificial intelligence system. Radiology. 2020;296(3):E166-E72..
These studies provide examples of the potential application of AI and DL in medical care. Nevertheless, while this technology holds great promise7Mintz Y, Brodie R. Introduction to artificial intelligence in medicine. Minim Invasive Ther Allied Technol. 2019;28(2):73-81., this technology has not seen widespread clinical application in medicine8Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-58.. The application of AI to dentistry has been studied, offering intriguing possibilities, and today the introduction of AI in clinical dentistry is on the horizon.
Application of AI to Dentistry
Several reviews have examined the application of AI to dentistry. Park and Park9Park WJ, Park JB. History and application of artificial neural networks in dentistry. Eur J Dent. 2018;12(4):594-601. emphasize that AI in healthcare offers to aid in decision-making, thereby allowing non-specialists to function as specialists in different fields. While the potential application of AI to different healthcare disciplines would seem limitless, most of the work has been in diagnosis and identification of risk and outcomes. Another review of the application of AI to dentistry tallied the potential applications that have begun to be evalued in a variety of dental specialties (adopted from Machoy et al.10Machoy ME, Szyszka-Sommerfeld L, Vegh A, Gedrange T, Wozniak K. The ways of using machine learning in dentistry. Adv Clin Exp Med. 2020;29(3):375-84.). These include:
Orthodontics:
- decision-making regarding the need for tooth extraction.
- prediction of tooth size of unerupted teeth.
- management of impacted canine teeth.
- cephalometric diagnosis.
- production of skeletal malocclusion related to mandibular morphology.
Restorative dentistry and prosthodontics:
- predicting restoration longevity.
- color matching for esthetic restorations.
- design of removable partial dentures.
- predicting the outcome of tooth-whitening procedures.
Periodontology and oral medicine:
- diagnosis and classification of periodontal disease.
- use of immunologic parameters to diagnosis aggressive periodontitis.
- classification of halitosis based on identification of periodontal pathogens in saliva.
- predicting recurrence of aphthous ulcers.
Temporomandibular joint disorders (TMD):
- use of magnetic resonance imaging to determine the prognosis of TMD.
- use of screening questions to aid in treatment of TMD.
- identification of subgroups of internal derangement of the temporomandibular joint.
Endodontics:
- location of accessory canals.
- detection of periapical radiolucencies11Endres MG, Hillen F, Salloumis M, Sedaghat AR, Niehues SM, Quatela O, et al. Development of a deep learning algorithm for periapical disease detection in dental radiographs. Diagnostics (Basel). 2020;10(6)..
Oral and Maxillofacial Surgery:
- detection of root fractures.
- clinical decision support for treatment with dental implants.
Oral Cancer:
- prognosis of oral cancer considering lesion histology and genetic characteristics.
- assessment of hyper-nasal speech following treatment of oral/oropharyngeal cancer.
- risk assessment for oral cancer.
Until recently, AI applications in clinical dentistry have been limited to research studies, but commercial applications that will impact clinical care are now being introduced. This is occurring primarily in the field of oral and maxillofacial radiology. This practical application is logical because imaging is essential to diagnosis and treatment of dental diseases and images are collected over time (allowing for assessment of disease progression). Further, the most common dental diseases (caries and periodontal diseases) occur throughout the world, so databases are available for ML. These large databases are needed for development and training of the system to recognize deviations from normal.
Why have AI applications not been adopted by the dental profession? The reasons include lack of availability of data due to patient privacy concerns, as well as lack of completeness of patient records, difficulty in defining the “gold standard”, i.e., what is normal and what is disease, and the available outcome provides only some of the data needed to develop a complete approach to treatment. The next decade will determine if the promise offered by application of AI in dentistry can be realized, which ultimately will result in improved care, for a greater number of individuals, and at a reduced cost.
A review of the application of AI for dental imaging suggest that the most common uses for AI in dental radiology will be for cephalometric analysis, identification of osteoporosis, identification of cysts and tumors of the jaws, as well as identification of periodontal and periapical disease12Hung K, Montalvao C, Tanaka R, Kawai T, Bornstein MM. The use and performance of artificial intelligence applications in dental and maxillofacial radiology: A systematic review. Dentomaxillofac Radiol. 2020;49(1):20190107..
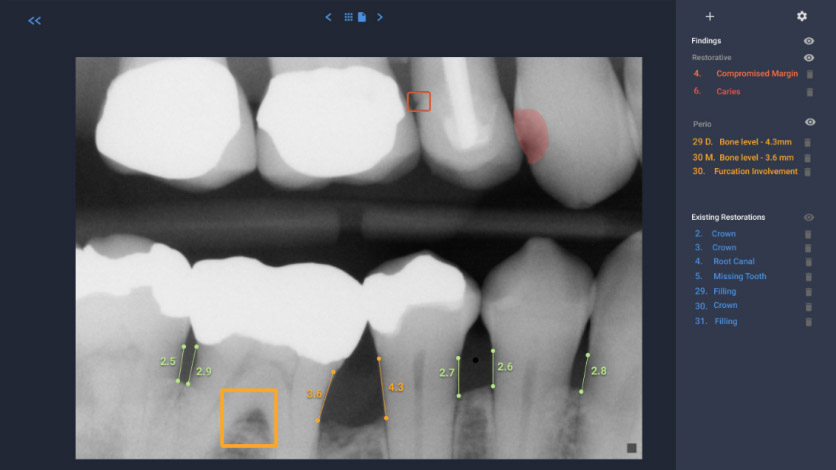
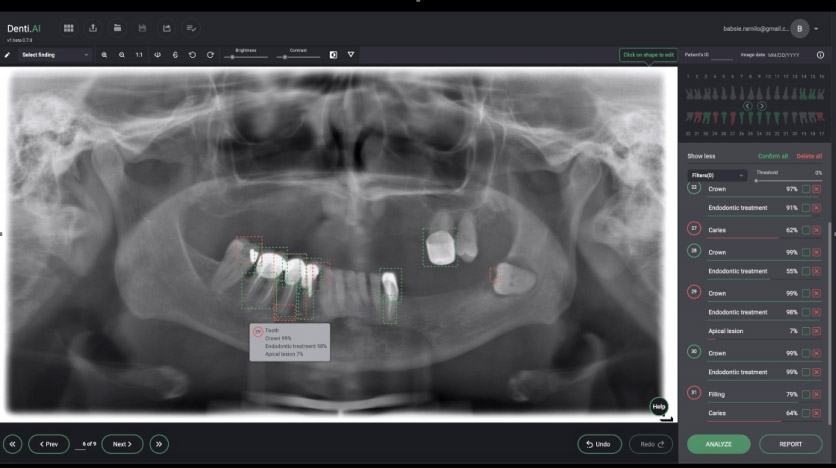
It appears that the broader introduction of AI into clinical dentistry will occur with interpretation of two-dimensional radiographs. Several start-up companies have developed systems to identify the anatomy, existing restorations and abnormal findings/pathology on bitewing and periapical films, as well as panoramic radiographs. (see Figures 2 and 3).
- Figure 2
- Figure 3
These programs will scan radiographs, and can identify the anatomy [i.e., tooth number, crowns, cementoenamel junction, root length, distance from the cementoenamel junction (CEJ) to the crest of the alveolar bone], as well as abnormal/pathologic findings (i.e., periodontal disease as an indicated by increased distance from the CEJ to the alveolar crest, caries, the presence of a periapical radiolucency and root fractures). This identification can serve as an internal check on the clinician’s assessment of the radiographs, and it is claimed, thereby free up time to allow the dentist to spend more time with patients. From the perspective of large multi-practice organizations (i.e., dental service organizations), this technology can also enhance quality control across multiple offices. It is also expected that dental insurance companies will utilize this technology to assure that adequate documentation (quality of the radiographs) is provided, and determine if the request for benefits is within guidelines.
The application of AI in dentistry for analysis of 3-dimensional imaging also is on the horizon13Hung K, Yeung AWK, Tanaka R, Bornstein MM. Current applications, opportunities, and limitations of ai for 3d imaging in dental research and practice. Int J Environ Res Public Health. 2020;17(12).. The expansion of the use of three-dimensional imaging, specifically cone beam computed tomography (CBCT), offers intriguing opportunities for the application of AI. The much greater amount of information contained in CBCT scans require more of the clinician’s time to fully analyzed the images. In this case, the use of AI will certainly improve efficiency and clinical accuracy.
There are, however, challenges. These include hesitancy of providers to adopt new approaches to practice and patient management. This is also the concern regarding the accuracy of the programs, as research reports describing the accuracy and validity of these programs are generally lacking. Another concern is cost and reimbursement.
There are several companies that now offer AI related to radiographic interpretation. Websites are provided below, which provide more information about the services that are offered. (No endorsement of any of these companies should be implied).
| Company | Website |
|---|---|
| Overjet | www.overjet.ai |
| Denti | www.denti.ai |
| VideaHealth | www.videa.ai |
| Pearl | www.hellopearl.com |
| Orca | www.orca-dental.ai |
| Retrace | www.retrace.ai |
The introduction of AI in dentistry is now gaining momentum, especially in these times of re-imagining clinical care and considering new ways to engage patients. While questions remain, a tipping point seems to have been reached that will bring AI into routine dental care. A review of potential applications and benefits suggest that the value added by the introduction of AI systems for radiographic interpretation in dental offices will provide the following:
- The identification of deviations from normal, specifically dental and osseous pathology. As such, this serves as a quality control measure. The findings will undoubtedly need to be matched with clinical data.
- Greater ease of tracking changes in radiographs over time.
- Improved efficiency within the practice. The findings can be linked to practice management software, and insurance claims can even be generated at the conclusion of the patient visit.
- Integration of medical and dental practices will be enhanced. This will depend in part on linkage of medical and dental electronic patents records. With this linkage dental providers will be able to see patient characteristics that can impact dental disease and treatment, i.e., diagnosis of diabetes, smoking status, and smoking history. Conversely, medical providers can have access to information in the dental record that may impact chronic disease, i.e., severity of periodontal disease.
- Improved patient relations as the introduction of these systems will build patient/provider trust.
- Introduction of these systems will differentiate practices, with a focus on adoption of new technology.
- Potential exists for using these systems to help educate students and younger members of the profession in radiographic interpretation.
All oral health care providers should be informed about the available (commercial) systems and understand what each can offer. In the ideal, these systems should improve the quality of care in a time-efficient manner.
Legends for Figures
Figure 1. Fundus photography of the retina. Fundus photography provides a visual image of the internal surface of the eye (retina). This technique is used to identify abnormalities associated with a variety of eye disorders, including diabetic retinopathy. AI can be used to identify these abnormalities when a specialist in not available. The top photograph is a normal retina and changes associate with diabetic retinopathy are seen in the lower photograph. The complex microvascular anatomy is well suited to analysis by AI.
Figure 2. Bitewing radiograph analyzed by an AI system identifies deviations from normal. In this system restorative findings (compromised margin of a restoration, dental caries), periodontal findings (reduced height of alveolar bone, furcation involvement) as well as existing restorations, are noted to the right. Image courtesy of Dr. Wardah Inam (Overjet.ai).
Figure 3. Panoramic radiograph analyzed by an AI system identifies deviations from normal. In this system deviations are listed by tooth number to the right. The percentages reflect the probability of that finding being present. Image courtesy of Dr. Eric Pulver (Denti.ai).
References
- 1.Zimmerli B, Jeger F, Lussi A. Bleaching of nonvital teeth. A clinically relevant literature review. Schweiz Monatsschr Zahnmed 2010;120(4):306-20.
- 2.Brunton PA, Burke FJT, Sharif MO et al. Contemporary dental practice in the UK in 2008: Aspects of direct restorations, endodontics, and bleaching. Br Dent J 2012;212:63-7.
- 3.Demarco FF, Conde MCM, Ely C et al. Preferences on vital and nonvital tooth bleaching: A survey among dentists from a city of Southern Brazil. Braz Dent J 2013;24:527-31.
- 4.Kahler B. Present status and future directions – Managing discoloured teeth. Int Endod J 2022: Feb 21. doi: 10.1111/iej.13711.
- 5.Nutting EB, Poe GS. Chemical bleaching of discolored endodontically treated teeth. Dent Clin N Am 1967;11:655-62.
- 6.Pallarés-Serrano A, Pallarés-Serrano S, Pallarés-Serrano A, Pallarés-Sabater A. Assessment of Oxygen Expansion during Internal Bleaching with Enamel and Dentin: A Comparative In Vitro Study. Dent J 2021;9:98. https://doi.org/10.3390/dj9090098.
- 7.Estay J, Angel P, Bersezio C et al. The change of teeth color, whiteness variations and its psychosocial and self-perception effects when using low vs. high concentration bleaching gels: a one-year follow-up. BMC Oral Health 2020;20(1):255. doi: 10.1186/s12903-020-01244-x.
- 8.Haywood VB, Bergeron BE. Bleaching and the Diagnosis of Internal Resorption. Jul 24, 2018. https://decisionsindentistry.com/article/bleaching-and-the-diagnosis-of-internal-resorption/
- 9.Abbott P, Heah SY. Internal bleaching of teeth: an analysis of 255 teeth. Aust Dent J 2009;54(4):326-33. https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1834-7819.2009.01158.x.
- 10.Sakalli B, Basmaci F, Dalmizrak O. Evaluation of the penetration of intracoronal bleaching agents into the cervical region using different intraorifice barriers. BMC Oral Health 2022;22(1):266. doi: 10.1186/s12903-022-02300-4.
- 11.American Association of Endodontists Clinical Practice Committee. Use of Silver Points. AAE Position Statement. https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/silverpointsstatement.pdf#:~:text=Silver%20points%20have%20been%20shown%20to%20corrode%20spontaneously,retreatment%20and%20replacement%20of%20the%20points%20with%20another.
- 12.Savadkouhi ST, Fazlyab M. Discoloration Potential of Endodontic Sealers: A Brief Review. Iran Endod J 2016;11(4):250-4. doi: 10.22037/iej.2016.20.
- 13.Ahmed HM, Abbott PV. Discolouration potential of endodontic procedures and materials: a review. Int Endod J 2012;45(10):883-97. doi: 10.1111/j.1365-2591.2012.02071.x.
- 14.Krastl G, Allgayer N, Lenherr P et al. Tooth discoloration induced by endodontic materials: a literature review. Dent Traumatol 2013;29(1):2-7. doi: 10.1111/j.1600-9657.2012.01141.x.
- 15.Ioannidis K, Mistakidis I, Beltes P, Karagiannis V. Spectrophotometric analysis of crown discoloration induced by MTA- and ZnOE-based sealers. J Appl Oral Sci 2013;21(2):138-44. doi: 10.1590/1678-7757201302254.
- 16.Santos LGPD, Chisini LA, Springmann CG et al. Alternative to Avoid Tooth Discoloration after Regenerative Endodontic Procedure: A Systematic Review. Braz Dent J 2018;29(5):409-18. doi: 10.1590/0103-6440201802132.
- 17.Watts A, Addy M. Tooth discoloration and staining: a review of the literature. Br Dent J 2001;190:309-16.
- 18.Pink tooth of Mummery. https://medical-dictionary.thefreedictionary.com/Pink+Tooth+of+Mummery.
- 19.Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int 1992:23:471-88. http://www.quintpub.com/userhome/qi/qi_23_7_haywood_6.pdf
- 20.Bersezio C, Ledezma P, Mayer C et al. Effectiveness and effect of non-vital bleaching on the quality of life of patients up to 6 months post-treatment: a randomized clinical trial. Clin Oral Investig 2018;22(9):3013-9. doi: 10.1007/s00784-018-2389-y.
- 21.Popescu AD, Purcarea MV, Georgescu RV et al. Vital and Non-Vital Tooth Bleaching Procedures: A Survey Among Dentists From Romania. Rom J Oral Rehab 2021;13(3):59-71. https://www.rjor.ro/wp-content/uploads/2021/10/VITAL-AND-NON-VITAL-TOOTH-BLEACHING-PROCEDURES-A-SURVEY-AMONG-DENTISTS-FROM-ROMANIA.pdf.
- 22.Zarean P, Zarean P, Ravaghi A et al. Comparison of MTA, CEM Cement, and Biodentine as Coronal Plug during Internal Bleaching: An In Vitro Study. Int J Dent 2020;2020:8896740. doi: 10.1155/2020/8896740.
- 23.Canoglu E, Gulsahi K, Sahin C et al. Effect of bleaching agents on sealing properties of different intraorifice barriers and root filling materials. Med Oral Patol Oral Cir Bucal 2012;17 (4):e710-5. http://www.medicinaoral.com/medoralfree01/v17i4/medoralv17i4p710.pdf
- 24.Amato A, Caggiano M, Pantaleo G, Amato M. In-office and walking bleach dental treatments on endodontically-treated teeth: 25 years follow-up. Minerva Stomatol 2018;67(6):225-30.
- 25.Lim MY, Lum SOY, Poh RSC et al. An in vitro comparison of the bleaching efficacy of 35% carbamide peroxide with established intracoronal bleaching agents. Int Endod J 2004;37(7):483-8. doi: 10.1111/j.1365-2591.2004.00829.x.
- 26.Yui KCK, Rodrigues JR, Mancini MNG et al. Ex vivo evaluation of the effectiveness of bleaching agents on the shade alteration of blood-stained teeth. Int Endod J 2008;41(6):485-92. doi: 10.1111/j.1365-2591.2008.01379.x.
- 27.Warren MA, Wong M, Ingram TA III. An in vitro comparison of bleaching agents on the crowns and roots of discolored teeth. J Endod 1990;16:463-7.
- 28.Rotstein I, Mor C, Friedman S. Prognosis of intracoronal bleaching with sodium perborate preparations in vitro: 1-year study. J Endod 1993;19:10-2.
- 29.Weiger R, Kuhn A, Lost C. In vitro comparison of various types of sodium perborate used for intracoronal bleaching of discolored teeth. J Endod 1994;20:338-41.
- 30.Amato M, Scaravilli MS, Farella M, Riccitiello F. Bleaching teeth treated endodontically: long-term evaluation of a case series. J Endod 2006;32:376-8.
- 31.Abou-Rass M. Long-term prognosis of intentional endodontics and internal bleaching of tetracycline-stained teeth. Compend Contin Educ Dent 1998;19:1034-44.
- 32.Anitua E, Zabalegui B, Gil J, Gascon F. Internal bleaching of severe tetracycline discolorations: four-year clinical evaluation. Quintessence Int 1990;21(10):783-8.
- 33.Gorr S-U, Brigman HV, Anderson JC, Hirsch EB. The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS ONE 2022;17(8): e0273504. https://doi.org/10.1371/journal.pone.0273504.
- 34.Peters SM, Hill NB, Halepas S. Oral manifestations of monkeypox: A report of two cases Journal of Oral and Maxillofacial Surgery (2022). https://doi.org/10.1016/j.joms.2022.07.147.
- 35.Portalatin A. Infectious disease experts call on dentists to monitor monkeypox symptoms: 6 notes. August 15, 2022. https://www.beckersdental.com/clinical-leadership-infection-control/39124-infectious-disease-experts-call-on-dentists-to-monitor-monkeypox-symptoms-6-notes.html?origin=DentalE&utm_source=DentalE&utm_medium=email&utm_content=newsletter&oly_enc_id=1694C1316967A3F.
- 36.CDC. Monkeypox. Non-Variola Orthopoxvirus and Monkeypox Virus Laboratory Testing Data. August 17, 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/2022-lab-test.html.
- 37.CDC. Monkeypox. Prevention. https://www.cdc.gov/poxvirus/monkeypox/prevention.html.
- 38.CDC. Monkeypox and smallpox vaccine guidance. Updated June 2, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/index.htm.
- 39.CDC. Monkeypox. ACAM Vaccine. https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/acam2000-vaccine.html.
- 40.CDC. Guidelines for Infection Control in Dental Health-Care Settings — 2003. https://www.cdc.gov/mmwr/PDF/rr/rr5217.pdf.
- 41.CDC. Infection Prevention and Control of Monkeypox in Healthcare Settings. Updated August 11, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- 42.CDC. Monkeypox. Information For Veterinarians. https://www.cdc.gov/poxvirus/monkeypox/veterinarian/index.html.
- 43.CDC. Monkeypox. Pets in the Home. https://www.cdc.gov/poxvirus/monkeypox/prevention/pets-in-homes.html.
- 44.Cheng YL, Musonda J, Cheng H, et al. Effect of surface removal following bleaching on the bond strength of enamel. BMC Oral Health 2019;19(1):50.
- 45.Monteiro D, Moreira A, Cornacchia T, Magalhães C. Evaluation of the effect of different enamel surface treatments and waiting times on the staining prevention after bleaching. J Clin Exp Dent 2017;9(5):e677-81.
- 46.Rezende M, Kapuchczinski AC, Vochikovski L, et al. Staining power of natural and artificial dyes after at-home dental bleaching. J Contemp Dent Pract 2019;20(4):424-7.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Ira-Lamster-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2021/02/ai-dental-medicine-2.jpg)