Oral Mucositis: Oral Impact, Sequelae and Management
Oral mucositis (OM) occurs as a complication of head and neck radiotherapy (HNR), chemotherapy (CT), and combination therapies.1Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 2007;5(9 Suppl 4):3-11.,2Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31.,3Bowen J, Al-Dasooqi N, Bossi P et al. The pathogenesis of mucositis: updated perspectives and emerging targets. Support Care Cancer 2019;27(10):4023-33. ,4Colella G, Boschetti CE, Vitagliano R et al. Interventions for the Prevention of Oral Mucositis in Patients Receiving Cancer Treatment: Evidence from Randomised Controlled Trials. Curr Oncol 2023;30(1):967-80. doi: 10.3390/curroncol30010074. This condition affects non-keratinized oral mucosa, including the buccal mucosa, soft palate, tongue, floor of the mouth and lips, and may also affect the pharyngeal area. In a systematic review of thirty-three studies (n>6,000), 80% of patients receiving HNR experienced OM, with a higher incidence of severe OM in patients receiving fractionated radiotherapy (RT).5Trotti A, Bellm LA, Epstein JB et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003;66(3):253-62. doi: 10.1016/s0167-8140(02)00404-8. In one study, more than 9 in 10 patients receiving HNR experienced OM,6Brown TJ, Gupta A. Management of Cancer Therapy-Associated Oral Mucositis. JCO Oncol Pract 2020;16(3):103-9. while 91% of patients in a second study experienced OM which was severe in two-thirds of them.7Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 2007;68(4):1110-20. doi: 10.1016/j.ijrobp.2007.01.053. With respect to CT, the incidence of OM is influenced by the chemotherapeutic and greater with higher doses and more frequent administration. Among patients receiving CT, for individuals with solid tumors the incidence of OM ranges from 20% to 40%, while in a systematic review of seventeen studies in children, mainly with solid and hematologic tumors, the mean incidence of OM was approximately 54% with almost 16% experiencing severe OM.6Brown TJ, Gupta A. Management of Cancer Therapy-Associated Oral Mucositis. JCO Oncol Pract 2020;16(3):103-9.,8Docimo R, Anastasio MD, Bensi C. Chemotherapy-induced oral mucositis in children and adolescents: a systematic review. Eur Arch Paediatr Dent 2022;23(4):501-11. doi: 10.1007/s40368-022-00727-5. Additionally, among patients receiving high-dose CT conditioning regimens prior to hematopoietic stem cell transplantation (HSCT) with or without total body irradiation (TBI), up to more than 90% and 30% to 50%, respectively, experience OM.9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. In a recent study (n=30), a prevalence of 80% was found for patients receiving HSCT.10Pereira TBF, Potter GS, Lima BMF et al. Oral Changes in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Cohort Study. J Oral Pathol Med 2025;54(5):351-9. doi: 10.1111/jop.13629.
Clinical Presentation
OM initially presents as erythema, with time to onset ranging from two to three weeks and one to two weeks, respectively, after initiation of HNR or CT, and from 3 to 4 days after the first treatment in conditioning regimens.6Brown TJ, Gupta A. Management of Cancer Therapy-Associated Oral Mucositis. JCO Oncol Pract 2020;16(3):103-9. While the mucosal inflammation can remain mild, OM typically develops further to an erosive phase and ulcerative lesions with pseudomembranous surfaces.1Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 2007;5(9 Suppl 4):3-11.,9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. When extensive, these lesions cause severe pain and can cause bleeding. OM development begins with the death of basal epithelial cells, which ensues rapidly once HNR or CT commences.1Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 2007;5(9 Suppl 4):3-11. The inflammatory process then involves the release of reactive oxygen species, gene expression and upregulation of multiple inflammatory pathways, and increased production of pro-inflammatory cytokines and other mediators.11Sonis ST, Elting LS, Keefe D et al. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer. International Society for Oral Oncology. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100(Suppl 9):1995-2025.,12Logan RM, Stringer AM, Bowen JM et al. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 2008;77:1139-45. OM lesions typically resolve two to four weeks after cancer therapy is completed. (Figure 1)
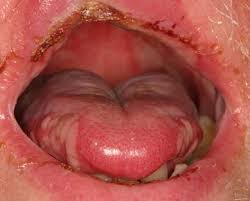
Figure 1. OM in patients who received HNR, showing normal, moderate delayed and severe delayed healing (left to right)
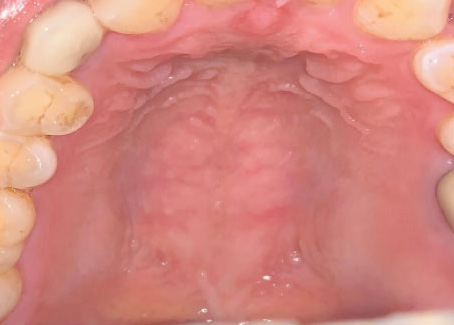
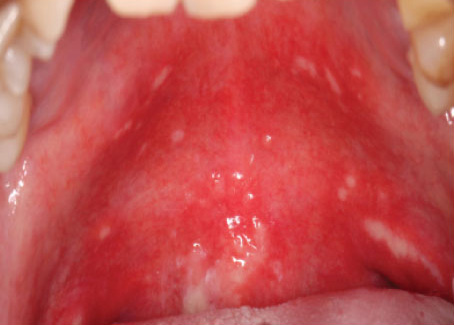
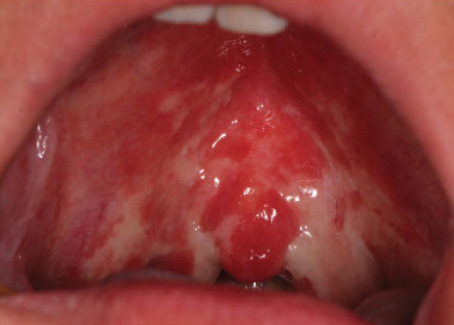
Source: Jiang R, Liu Y, Zhang H et al. Distinctive microbiota of delayed healing of oral mucositis after radiotherapy of nasopharyngeal carcinoma. Front Cell Infect Microbiol 2022;12:1070322. Copyright © 2022 Jiang, Liu, Zhang, Chen, Liu, Zeng, Nie, Chen and Tan.
Risk factors for OM
Risk factors include poor oral hygiene, dry mouth, and prior cancer therapy.13Wolff H, Zomorodbakhsch B, Schnizer M et al. Evaluation of patient management of (radio-) chemotherapy-caused mucositis with the goal of enhancing patient treatment. J Cancer Res Clin Oncol 2025;151(7):211. doi: 10.1007/s00432-025-06238-2. Furthermore, the incidence and severity of OM and delayed healing has been found to be associated with significant changes to the oral microbiome, including the role of anaerobic bacteria and increases in the levels of Actinobacteria and Veillonella; and, increases in aerobic microorganisms in patients receiving HNR.9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. ,14Jiang R, Liu Y, Zhang H et al. Distinctive microbiota of delayed healing of oral mucositis after radiotherapy of nasopharyngeal carcinoma. Front Cell Infect Microbiol 2022;12:1070322. Genetics is a significant risk factor, including polymorphisms for pro-inflammatory mediators (e.g., TNF-α), and regulation of enzymes and metabolites for chemotherapeutics.9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. ,15Sonis ST. Genomics, Personalized Medicine, and Supportive Cancer Care. Am Soc Clin Oncol Educ Book 2015;35:9-16. doi:10.14694/EdBook_AM.2015.35.9,16Bogunia-Kubik K, Polak M, Lange A. TNF polymorphisms are associated with toxic but not with aGVHD complications in the recipients of allogeneic sibling haematopoietic stem cell transplantation. Bone Marrow Transplantation 2003;32(6):617-22. doi: 10.1038/sj.bmt.1704200. Further, in a pilot study with adolescents and young adults, baseline levels of interleukin-1a and epidermal growth factor were found to be indicative of the likelihood of OM, and stress was related to OM scores.17Thornton CP, Perrin N, Kozachik S et al. Biobehavioral Influences of Stress and Inflammation on Mucositis in Adolescents and Young Adults with Cancer: Results from a Pilot Study. J Adolesc Young Adult Oncol 2023;12(3):340-48. doi: 10.1089/jayao.2022.0067. For CT, additional risk factors for OM and its severity include the specific chemotherapeutic and the regimen followed (dose level and frequency of administration).6Brown TJ, Gupta A. Management of Cancer Therapy-Associated Oral Mucositis. JCO Oncol Pract 2020;16(3):103-9.,9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. ,13Wolff H, Zomorodbakhsch B, Schnizer M et al. Evaluation of patient management of (radio-) chemotherapy-caused mucositis with the goal of enhancing patient treatment. J Cancer Res Clin Oncol 2025;151(7):211. doi: 10.1007/s00432-025-06238-2.
Sequelae
OM-associated pain can lead to difficulty eating, drinking, and swallowing. When pain is severe, reduced intake of foods and beverages, weight loss, inadequate oral hygiene, loss of sleep, fatigue, and poor quality of life may ensue.9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. ,13Wolff H, Zomorodbakhsch B, Schnizer M et al. Evaluation of patient management of (radio-) chemotherapy-caused mucositis with the goal of enhancing patient treatment. J Cancer Res Clin Oncol 2025;151(7):211. doi: 10.1007/s00432-025-06238-2.,18Elting LS, Keefe DM, Sonis ST et al. Burden of Illness Head and Neck Writing Committee. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008;113(10):2704-13. doi: 10.1002/cncr.23898.,19Hadjieva T, Cavallin-Ståhl E, Linden M, Tiberg F. Treatment of oral mucositis pain following radiation therapy for head-and-neck cancer using a bioadhesive barrier-forming lipid solution. Support Care Cancer 2014;22(6):1557-62. doi: 10.1007/s00520-014-2117-3. Paradoxically, poor nutrition is associated with a higher incidence of OM. Severe pain and lack of nutrition can result in patients being hospitalized for feeding, and necessitate pauses in cancer therapy.7Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 2007;68(4):1110-20. doi: 10.1016/j.ijrobp.2007.01.053.,20Pulito C, Cristaudo A, Porta C et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 2020;39(1):210.,21Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am 2008;52(1):61-77. (Table 1) In one systematic review, among three studies (n=700) 16% of patients receiving HNR were hospitalized with severe OM, while across five studies included in the same systematic review (n~1300), severe OM resulted in cancer therapy being paused in 11% of patients.7Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 2007;68(4):1110-20. doi: 10.1016/j.ijrobp.2007.01.053. Loss of integrity of the oral mucosa also places patients at greater risk for local infections and systemic infections that can be life-threatening.9Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281. ,22Hong BY, Sobue T, Choquette L et al.: Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019;7(1):66.,23Sonis ST. Oral mucositis in head and neck cancer: risk, biology, and management. Am Soc Clin Oncol Educ Book, 2013. doi: 10.14694/EdBook_AM.2013.33.e236.,24McCann S, Schwenkglenks M, Bacon P et al. The Prospective Oral Mucositis Audit: relationship of severe oral mucositis with clinical and medical resource use outcomes in patients receiving high‐dose melphalan or BEAM‐conditioning chemotherapy and autologous SCT. Bone Marrow Transplant 2009;43:141‐7. Rarely, severe OM may result in airway obstruction, necessitating intubation or tracheostomy.25Tsuboi K, Tsuboi N, Sakamoto K et al. Life-threatening oral mucositis following chemotherapy in a pediatric patient. Clin Case Rep 2021;9(6):e04356. doi: 10.1002/ccr3.4356.
| Table 1. OM severity and functioning | |
|---|---|
| Grade 1 | Asymptomatic or mild; intervention not indicated |
| Grade 2 | Moderate pain or ulcer that does not interfere with oral intake; modified diet indicated |
| Grade 3 | Severe pain interfering with oral intake |
| Grade 4 | Life-threatening consequences; urgent intervention indicated |
| Grade 5 | Death |
Source: U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, page 37. https://dctd.cancer.gov/research/ctep-trials/for-sites/adverse-events/ctcae-v5-5x7.pdf.
The Role of Oral Health Professionals
Dental professionals play a key role in helping patients manage their oral health-related treatment needs and issues prior to and during cancer therapy, and are considered part of the oncology team.26National Institute of Health. National Cancer Institute. Oral Complications of Cancer Therapies (PDQ®)–Health Professional Version. Updated February 16, 2024. https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq. This is especially important given the incidence, presentation, and risk factors for OM and its severity - as well as other cancer therapy sequelae beyond the scope of this article, and the associated impacts on oral and systemic health, and quality of life. In addition, in a recent systematic review of thirty-nine studies (including twenty-five randomized controlled trials (RCT) and almost three thousand patients), the researchers concluded that dental hygiene protocols followed during cancer therapy reduced the severity of OM and associated pain.27de Lima Martins JO, Carlos ACAM, Costa GAJ et al. Oral hygiene protocols reduce the severity and incidence of oral mucositis during antineoplastic treatment: a systematic review and meta-analysis of randomized and non-randomized clinical trials. Support Care Cancer 2023;31(8):480. doi: 10.1007/s00520-023-07858-5. Interprofessional collaboration across disciplines within the patient’s oncology team is essential during treatment planning and for the integration of patient care; and, dental professionals with no/limited experience in treating this patient population can also consult with, or refer patients to, colleagues with more experience.28American Dental Association. Cancer Therapies and Dental Considerations. Updated August 30, 2022. https://www.ada.org/resources/ada-library/oral-health-topics/cancer-therapies-and-dental-considerations.,29Samim F, Epstein JB, Zumsteg ZS et al. Oral and dental health in head and neck cancer survivors. Cancers Head Neck 2016;1:14. doi: 10.1186/s41199-016-0015-8.,30Kamel AHM, AlKindi F, AlHarrasi R, AlKindi N. The Role of Dental Oncology in Cancer Care: a Critical Component of Comprehensive Treatment, Education, and Interdisciplinary Collaboration- a Narrative Review. J Cancer Educ 2025. doi: 10.1007/s13187-025-02639-6. In general, patients should see a dental professional four weeks prior to the initiation of cancer therapy and, if oral surgery is required, this should be performed at least 2 weeks prior to cancer therapy.28American Dental Association. Cancer Therapies and Dental Considerations. Updated August 30, 2022. https://www.ada.org/resources/ada-library/oral-health-topics/cancer-therapies-and-dental-considerations.,29Samim F, Epstein JB, Zumsteg ZS et al. Oral and dental health in head and neck cancer survivors. Cancers Head Neck 2016;1:14. doi: 10.1186/s41199-016-0015-8. However, 4 in 5 responding dentists in one survey reported that there was insufficient time prior to HNR to render indicated dental treatment, further noting that the main reason for this was poor communication between healthcare professionals.31Patel Y, Bahlhorn H, Zafar S et al. Survey of Michigan dentists and radiation oncologists on oral care of patients undergoing head and neck radiation therapy. J Mich Dent Assoc 2012;94(7):34-45.
Prior to Cancer Therapy
Patients should receive a thorough oral evaluation prior to cancer therapy to determine their oral health status, advice on the importance of good oral hygiene, detailed oral hygiene instructions (OHI), and indicated treatment.31Patel Y, Bahlhorn H, Zafar S et al. Survey of Michigan dentists and radiation oncologists on oral care of patients undergoing head and neck radiation therapy. J Mich Dent Assoc 2012;94(7):34-45. OHI should emphasize brushing with an ultra-soft/soft toothbrush and a mildly-flavored or non-flavored fluoride toothpaste, performed twice-daily (or more frequently if indicated), as well as patient-appropriate interdental cleaning that will not traumatize periodontal tissue and adjunctive rinsing with a saline or antimicrobial mouthrinse.28American Dental Association. Cancer Therapies and Dental Considerations. Updated August 30, 2022. https://www.ada.org/resources/ada-library/oral-health-topics/cancer-therapies-and-dental-considerations.,32Hong CHL, Gueiros LA, Fulton JS et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019;27(10):3949-67. doi:10.1007/s00520-019-04848-4. If patients wear dentures, denture cleaning should be reinforced along with advice to soak these in antimicrobial solutions; and, as needed during cancer therapy, patients should avoid/limit wearing of dentures e.g., when OM is present. Patients should also be advised on diet and other lifestyle choices that will help to preserve oral health and reduce the risk of irritation during cancer therapy (and OM), such as avoiding foods and beverages that are spicy, acidic, contain alcohol or added sugars, as well as on avoiding crunchy/hard foods and tobacco use. Additionally, patients need to be advised on OM and other potential sequelae during cancer therapy, and to seek care if these occur. Of note, while the focus of this article is OM, a caries control program that includes in-office fluoride applications and home use of prescription-level fluoride toothpaste started before cancer therapy begins helps to prevent and manage dental caries in advance.25Tsuboi K, Tsuboi N, Sakamoto K et al. Life-threatening oral mucositis following chemotherapy in a pediatric patient. Clin Case Rep 2021;9(6):e04356. doi: 10.1002/ccr3.4356.
Examples of treatment include the extraction of non-restorable (‘hopeless’) teeth, management of caries lesions, periodontal debridement, elimination of acute oral infections and loci of infection (chronic), the smoothing/treatment of tooth fractures/chips as well as rough restoration and denture surfaces to reduce the risk of oral irritation, and orthodontic appliance removal.26National Institute of Health. National Cancer Institute. Oral Complications of Cancer Therapies (PDQ®)–Health Professional Version. Updated February 16, 2024. https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq. A decision on the priorities/extent of dental treatment is informed by the urgency of cancer therapy, health status and specifics of the planned cancer therapy and dental treatment, thus requiring interprofessional collaboration. For example, the risks associated with oral surgery are greater after HNR compared to CT; therefore, oral foci of chronic infection that cannot be otherwise resolved may require an extraction in a patient who will receive HNR but may not if receiving CT alone.26National Institute of Health. National Cancer Institute. Oral Complications of Cancer Therapies (PDQ®)–Health Professional Version. Updated February 16, 2024. https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq.,33Spijkervet FKL, Schuurhuis JM, Stokman MA, Witjes MJH, Vissink A. Should oral foci of infection be removed before the onset of radiotherapy or chemotherapy? Oral Dis 2021;27(1):7-13. doi: 10.1111/odi.13329. As later determined, patients may need dental treatment during cancer therapy.
Palliative care for OM
Palliative care for patients with OM may involve the use of ice chips/cold water to temporarily cool the affected area, rinses, barrier protection, local and systemic analgesics, and other treatments.34PDQ Supportive and Palliative Care Editorial Board. Oral Complications of Cancer Therapies (PDQ®): Health Professional Version. Updated February 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK65881.6/. Of importance, patients are known to perceive OM as being more painful and detrimental to their quality of life than do healthcare professionals.23Sonis ST. Oral mucositis in head and neck cancer: risk, biology, and management. Am Soc Clin Oncol Educ Book, 2013. doi: 10.14694/EdBook_AM.2013.33.e236.,35Kanagalingam J, Wahid MIA, Lin JC et al. Patient and oncologist perceptions regarding symptoms and impact on quality-of-life of oral mucositis in cancer treatment: results from the Awareness Drives Oral Mucositis PercepTion (ADOPT) study. Support Care Cancer 2018;26(7):2191-200. doi: 10.1007/s00520-018-4050-3. Selection is based on the individual patient’s needs, general and patient-specific contraindications and relative contraindications, and is further beyond the scope of this article.
Saline and Sodium Bicarbonate Rinses
Rinses recommended for use 4 to 6 times per day include one containing 0.9% sodium chloride, or sodium bicarbonate, or both ingredients (e.g., a rinse containing ½ teaspoon salt, ½ teaspoon baking soda and 8 fl oz water).2Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31.,36Cancer Care. Managing oral mucositis. https://media.cancercare.org/publications/original/359-oral_mucositis.pdf
Barrier Protection – mucoadhesive rinses, sprays, and gels
Protective coatings isolate ulcerations and irritations from the oral environment, relieving mild to moderate OM. Mucoadhesive rinses recommended include bismuth subsalicylate (Kaopectate), and Mugard which contains glycerin, a carbomer and other ingredients and has been found to effectively reduce OM-associated pain.35Kanagalingam J, Wahid MIA, Lin JC et al. Patient and oncologist perceptions regarding symptoms and impact on quality-of-life of oral mucositis in cancer treatment: results from the Awareness Drives Oral Mucositis PercepTion (ADOPT) study. Support Care Cancer 2018;26(7):2191-200. doi: 10.1007/s00520-018-4050-3.,37Allison RR, Ambrad AA, Arshoun Y et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer 2014;120(9):1433-40. A mucoadhesive spray (Episil) has also been shown to help relieve pain and to reduce OM severity, and locally applied mucoadhesive gels may help relieve discomfort.38Ito K, Tokura S, Takazawa I et al. Clinical investigation of use of Episil® oral solution in oral mucositis during radiotherapy for head and neck cancer. Heliyon 2023;9(6):e15869. doi: 10.1016/j.heliyon.2023.e15869.,39Wei J, Wu J, Wang H et al. A Bioadhesive Barrier-Forming Oral Liquid Gel Improved Oral Mucositis and Nutritional Status in Patients With Head and Neck Cancers Undergoing Radiotherapy: A Retrospective Single Center Study. Front Oncol 2021;11:617392. doi: 10.3389/fonc.2021.617392. ‘Magic Mouthwash’ is a compounded mucoadhesive analgesic rinse; there is considerable controversy regarding its use, partially due to varying formulations (e.g., some contain an anti-fungal agent and/or hydrocortisone). Based on limited data from a phase III study, a formulation containing magnesium aluminum hydroxide (Maalox), lidocaine and diphenhydramine (an antihistamine) was more effective than placebo, while in a second study a similar formulation, sodium chloride and sodium bicarbonate, and chlorhexidine rinses were not found to offer any adjunctive value in an oral hygiene regimen.40Miller RC, Le-Rademacher J, Sio TTW. A Phase III, Randomized Double-Blind Study of Doxepin Rinse versus Magic Mouthwash versus Placebo in the Treatment of Acute Oral Mucositis Pain in Patients Receiving Head and Neck Radiotherapy with or without Chemotherapy (Alliance A221304). Int J Rad Oncol Biol Phys 2016;96(5):938.,41Dodd MJ, Dibble SL, Miaskowski C et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90(1):39-47. doi: 10.1067/moe.2000.105713.
Other rinses
Supersaturated calcium phosphate rinses moisturize the oral mucosa and are recommended adjuncts for patients with OM. Caphosol is indicated for OM induced by HNR or high-level CT, and was found to effectively reduce the duration and severity of OM in 24 of 30 studies included in a review and in one RCT reduced the incidence, severity and duration of OM in patients receiving HSCT.42Quinn B. Efficacy of a supersaturated calcium phosphate oral rinse for the prevention and treatment of oral mucositis in patients receiving high-dose cancer therapy: a review of current data. Eur J Cancer Care (Engl) 2013;22(5):564-79. doi: 10.1111/ecc.12073.,43Waśko-Grabowska A, Rzepecki P, Oborska S et al. Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc 2011;43(8):3111-3. doi: 10.1016/j.transproceed.2011.08.053. Other supersaturated calcium phosphate rinses indicated for patients with OM include NeutraSal and SalivaMax, which are delivered as a powder and reconstituted with water immediately before rinsing.44Dass K, Armstrong J, Goodwin J et al. Efficacy of NeutraSal (Supersaturated Calcium Phosphate Rinse) in the Prevention and Treatment of Chemotherapy-induced or Radiotherapy-induced Oral Mucositis. ASTRO 2012 Study Registry Abstract, May 2012.,45Brock J, Morris C, Hotze K, Pikkula B. Supersaturated Calcium Phosphate Rinse vs. Standard of Care for Mitigating Mucositis in Head and Neck Chemoradiation. J Cancer Ther 2018;9:262-7. https://doi.org/10.4236/jct.2018.93023. Additionally, research on rinses containing zinc chloride or zinc sulfate/polyherbal suggests that these may be effective in reducing the severity of OM and associated pain.46Mohammadi F, Oshvandi K, Kamallan SR et al. Effectiveness of sodium bicarbonate and zinc chloride mouthwashes in the treatment of oral mucositis and quality of life in patients with cancer under chemotherapy. Nurs Open 2022;9(3):1602-11. doi: 10.1002/nop2.1168. ,47Oshvandi K, Vafaei SY, Kamallan SR et al. Effectiveness of zinc chloride mouthwashes on oral mucositis and weight of patients with cancer undergoing chemotherapy. BMC Oral Health 2021;21(1):364. doi: 10.1186/s12903-021-01706-w. ,48Yarom N, Hovan A, Bossi P et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: vitamins, minerals, and nutritional supplements. Support Care Cancer 2019;27(10):3997-4010.
Analgesic agents
Topical analgesic pastes, gels and sprays containing 20% benzocaine or 2% lidocaine and solutions containing diphenhydramine or dyclonine hydrochloride can relieve mild to moderate pain.35Kanagalingam J, Wahid MIA, Lin JC et al. Patient and oncologist perceptions regarding symptoms and impact on quality-of-life of oral mucositis in cancer treatment: results from the Awareness Drives Oral Mucositis PercepTion (ADOPT) study. Support Care Cancer 2018;26(7):2191-200. doi: 10.1007/s00520-018-4050-3. Topical corticosteroid ointments are a further option for palliative relief in patients with mild to moderate OM.28American Dental Association. Cancer Therapies and Dental Considerations. Updated August 30, 2022. https://www.ada.org/resources/ada-library/oral-health-topics/cancer-therapies-and-dental-considerations. (Table 2)
Vitamins, minerals, and nutritional supplements have been suggested as ‘natural options’ for the management and prevention of OM, with a recent systematic review providing insights into their use.48Yarom N, Hovan A, Bossi P et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: vitamins, minerals, and nutritional supplements. Support Care Cancer 2019;27(10):3997-4010. The researchers concluded that limited/contradictory evidence precluded providing guidelines for several options, including oral or systemic zinc; vitamin E; selenium, folinic acid and calcitriol.
| Table 2. Recommended options for Palliative Care of OM (mild/moderate) | |
|---|---|
| Local cooling effect | Ice chips, cold water, cold rinses |
| Cleansing rinses |
|
| Barrier protection | Mucoadhesive rinses: Kaopectate, Mugard, magic mouthwash; Mucoadhesive sprays and gels |
| Supersaturated calcium phosphate rinses | Caphosol, Neutrasal, SalivaMax |
| Topical analgesics |
|
Patients with severe OM require more complex care for treatment of this potentially life-threatening and treatment-complicating condition. The oncology team at their treatment center may recommend 0.2% morphine rinse for patients experiencing moderate pain.2Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31. For patients with severe OM, options include systemic corticosteroids, nonsteroidal anti-inflammatory drugs, and opioids.21Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am 2008;52(1):61-77.,35Kanagalingam J, Wahid MIA, Lin JC et al. Patient and oncologist perceptions regarding symptoms and impact on quality-of-life of oral mucositis in cancer treatment: results from the Awareness Drives Oral Mucositis PercepTion (ADOPT) study. Support Care Cancer 2018;26(7):2191-200. doi: 10.1007/s00520-018-4050-3. Patients with severe OM may require additional treatment.
Other considerations – preventive therapies
Within the medical oncology setting, placing a cold substance (typically ice or cold water) intraorally for 30 minutes relieves pain while certain chemotherapeutics are administered, and is supported by a recent systematic review (36 studies; 2020) in which reductions in the incidence of severe OM were found.2Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31.,49Correa MEP, Cheng KKF, Chiang K et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020;28(5):2449-56. doi: 10.1007/s00520-019-05217-x. A few preventive therapies are available in the medical setting, including keratinocyte growth factor-1 (palmiferin) which is FDA-approved, administered intravenously, and recommended and supported for OM prevention in adults receiving conditioning regimens and TBI as part of HSCT.2Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31.,20Pulito C, Cristaudo A, Porta C et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 2020;39(1):210.,50Logan RM, Al-Azri AR, Bossi P et al; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020;28(5):2485-98. doi: 10.1007/s00520-019-05170-9.,51Riley P, Glenny AM, Worthington HV et al. Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors. Cochrane Database Syst Rev 2017;11(11):CD011990. doi: 10.1002/14651858.CD011990.pub2. Low-level laser therapy is also recommended for the same adult patient group, as well as adults receiving HNR plus CT or HNR alone (caution: without exposing cancer cells to the laser beam).2Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31. In patients receiving HNR oral glutamine is a further option, and honey has been proposed as a preventive in patients receiving HNR or combination therapy.
Conclusions
OM, a common complication of HNR, CT and conditioning regimens, is debilitating, impacts quality-of-life, potentially life-threatening and can interrupt cancer therapy. All patients should see a dental professional prior to cancer therapy for a full oral health evaluation, advice and needed treatment to reduce the risk of OM and other therapy-related complications, as well as potential complications associated with dental treatment during/after cancer therapy. Dental professionals should advise patients on OM as an oral complication of cancer therapy (and on other oral complications), OHI, and lifestyle factors playing a role in OM and oral irritation. Patients should also be advised on the need for completing indicated dental treatment, and dental professionals can also provide advice and palliative treatment options for pain relief from OM – including sending patients over to their medical oncology setting. Active involvement and collaboration is essential for optimal care.
References
- 1.Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 2007;5(9 Suppl 4):3-11.
- 2.Elad S, Cheng KKF, Lalla RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423-31.
- 3.Bowen J, Al-Dasooqi N, Bossi P et al. The pathogenesis of mucositis: updated perspectives and emerging targets. Support Care Cancer 2019;27(10):4023-33.
- 4.Colella G, Boschetti CE, Vitagliano R et al. Interventions for the Prevention of Oral Mucositis in Patients Receiving Cancer Treatment: Evidence from Randomised Controlled Trials. Curr Oncol 2023;30(1):967-80. doi: 10.3390/curroncol30010074.
- 5.Trotti A, Bellm LA, Epstein JB et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003;66(3):253-62. doi: 10.1016/s0167-8140(02)00404-8.
- 6.Brown TJ, Gupta A. Management of Cancer Therapy-Associated Oral Mucositis. JCO Oncol Pract 2020;16(3):103-9.
- 7.Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 2007;68(4):1110-20. doi: 10.1016/j.ijrobp.2007.01.053.
- 8.Docimo R, Anastasio MD, Bensi C. Chemotherapy-induced oral mucositis in children and adolescents: a systematic review. Eur Arch Paediatr Dent 2022;23(4):501-11. doi: 10.1007/s40368-022-00727-5.
- 9.Haverman TM, Raber-Durlacher JE, Rademacher WM et al. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm 2014:378281. doi: 10.1155/2014/378281.
- 10.Pereira TBF, Potter GS, Lima BMF et al. Oral Changes in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Cohort Study. J Oral Pathol Med 2025;54(5):351-9. doi: 10.1111/jop.13629.
- 11.Sonis ST, Elting LS, Keefe D et al. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer. International Society for Oral Oncology. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100(Suppl 9):1995-2025.
- 12.Logan RM, Stringer AM, Bowen JM et al. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 2008;77:1139-45.
- 13.Wolff H, Zomorodbakhsch B, Schnizer M et al. Evaluation of patient management of (radio-) chemotherapy-caused mucositis with the goal of enhancing patient treatment. J Cancer Res Clin Oncol 2025;151(7):211. doi: 10.1007/s00432-025-06238-2.
- 14.Jiang R, Liu Y, Zhang H et al. Distinctive microbiota of delayed healing of oral mucositis after radiotherapy of nasopharyngeal carcinoma. Front Cell Infect Microbiol 2022;12:1070322.
- 15.Sonis ST. Genomics, Personalized Medicine, and Supportive Cancer Care. Am Soc Clin Oncol Educ Book 2015;35:9-16. doi:10.14694/EdBook_AM.2015.35.9
- 16.Bogunia-Kubik K, Polak M, Lange A. TNF polymorphisms are associated with toxic but not with aGVHD complications in the recipients of allogeneic sibling haematopoietic stem cell transplantation. Bone Marrow Transplantation 2003;32(6):617-22. doi: 10.1038/sj.bmt.1704200.
- 17.Thornton CP, Perrin N, Kozachik S et al. Biobehavioral Influences of Stress and Inflammation on Mucositis in Adolescents and Young Adults with Cancer: Results from a Pilot Study. J Adolesc Young Adult Oncol 2023;12(3):340-48. doi: 10.1089/jayao.2022.0067.
- 18.Elting LS, Keefe DM, Sonis ST et al. Burden of Illness Head and Neck Writing Committee. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008;113(10):2704-13. doi: 10.1002/cncr.23898.
- 19.Hadjieva T, Cavallin-Ståhl E, Linden M, Tiberg F. Treatment of oral mucositis pain following radiation therapy for head-and-neck cancer using a bioadhesive barrier-forming lipid solution. Support Care Cancer 2014;22(6):1557-62. doi: 10.1007/s00520-014-2117-3.
- 20.Pulito C, Cristaudo A, Porta C et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 2020;39(1):210.
- 21.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am 2008;52(1):61-77.
- 22.Hong BY, Sobue T, Choquette L et al.: Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019;7(1):66.
- 23.Sonis ST. Oral mucositis in head and neck cancer: risk, biology, and management. Am Soc Clin Oncol Educ Book, 2013. doi: 10.14694/EdBook_AM.2013.33.e236.
- 24.McCann S, Schwenkglenks M, Bacon P et al. The Prospective Oral Mucositis Audit: relationship of severe oral mucositis with clinical and medical resource use outcomes in patients receiving high‐dose melphalan or BEAM‐conditioning chemotherapy and autologous SCT. Bone Marrow Transplant 2009;43:141‐7.
- 25.Tsuboi K, Tsuboi N, Sakamoto K et al. Life-threatening oral mucositis following chemotherapy in a pediatric patient. Clin Case Rep 2021;9(6):e04356. doi: 10.1002/ccr3.4356.
- 26.National Institute of Health. National Cancer Institute. Oral Complications of Cancer Therapies (PDQ®)–Health Professional Version. Updated February 16, 2024. https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq.
- 27.de Lima Martins JO, Carlos ACAM, Costa GAJ et al. Oral hygiene protocols reduce the severity and incidence of oral mucositis during antineoplastic treatment: a systematic review and meta-analysis of randomized and non-randomized clinical trials. Support Care Cancer 2023;31(8):480. doi: 10.1007/s00520-023-07858-5.
- 28.American Dental Association. Cancer Therapies and Dental Considerations. Updated August 30, 2022. https://www.ada.org/resources/ada-library/oral-health-topics/cancer-therapies-and-dental-considerations.
- 29.Samim F, Epstein JB, Zumsteg ZS et al. Oral and dental health in head and neck cancer survivors. Cancers Head Neck 2016;1:14. doi: 10.1186/s41199-016-0015-8.
- 30.Kamel AHM, AlKindi F, AlHarrasi R, AlKindi N. The Role of Dental Oncology in Cancer Care: a Critical Component of Comprehensive Treatment, Education, and Interdisciplinary Collaboration- a Narrative Review. J Cancer Educ 2025. doi: 10.1007/s13187-025-02639-6.
- 31.Patel Y, Bahlhorn H, Zafar S et al. Survey of Michigan dentists and radiation oncologists on oral care of patients undergoing head and neck radiation therapy. J Mich Dent Assoc 2012;94(7):34-45.
- 32.Hong CHL, Gueiros LA, Fulton JS et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019;27(10):3949-67. doi:10.1007/s00520-019-04848-4.
- 33.Spijkervet FKL, Schuurhuis JM, Stokman MA, Witjes MJH, Vissink A. Should oral foci of infection be removed before the onset of radiotherapy or chemotherapy? Oral Dis 2021;27(1):7-13. doi: 10.1111/odi.13329.
- 34.PDQ Supportive and Palliative Care Editorial Board. Oral Complications of Cancer Therapies (PDQ®): Health Professional Version. Updated February 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK65881.6/.
- 35.Kanagalingam J, Wahid MIA, Lin JC et al. Patient and oncologist perceptions regarding symptoms and impact on quality-of-life of oral mucositis in cancer treatment: results from the Awareness Drives Oral Mucositis PercepTion (ADOPT) study. Support Care Cancer 2018;26(7):2191-200. doi: 10.1007/s00520-018-4050-3.
- 36.Cancer Care. Managing oral mucositis. https://media.cancercare.org/publications/original/359-oral_mucositis.pdf
- 37.Allison RR, Ambrad AA, Arshoun Y et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer 2014;120(9):1433-40.
- 38.Ito K, Tokura S, Takazawa I et al. Clinical investigation of use of Episil® oral solution in oral mucositis during radiotherapy for head and neck cancer. Heliyon 2023;9(6):e15869. doi: 10.1016/j.heliyon.2023.e15869.
- 39.Wei J, Wu J, Wang H et al. A Bioadhesive Barrier-Forming Oral Liquid Gel Improved Oral Mucositis and Nutritional Status in Patients With Head and Neck Cancers Undergoing Radiotherapy: A Retrospective Single Center Study. Front Oncol 2021;11:617392. doi: 10.3389/fonc.2021.617392.
- 40.Miller RC, Le-Rademacher J, Sio TTW. A Phase III, Randomized Double-Blind Study of Doxepin Rinse versus Magic Mouthwash versus Placebo in the Treatment of Acute Oral Mucositis Pain in Patients Receiving Head and Neck Radiotherapy with or without Chemotherapy (Alliance A221304). Int J Rad Oncol Biol Phys 2016;96(5):938.
- 41.Dodd MJ, Dibble SL, Miaskowski C et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90(1):39-47. doi: 10.1067/moe.2000.105713.
- 42.Quinn B. Efficacy of a supersaturated calcium phosphate oral rinse for the prevention and treatment of oral mucositis in patients receiving high-dose cancer therapy: a review of current data. Eur J Cancer Care (Engl) 2013;22(5):564-79. doi: 10.1111/ecc.12073.
- 43.Waśko-Grabowska A, Rzepecki P, Oborska S et al. Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc 2011;43(8):3111-3. doi: 10.1016/j.transproceed.2011.08.053.
- 44.Dass K, Armstrong J, Goodwin J et al. Efficacy of NeutraSal (Supersaturated Calcium Phosphate Rinse) in the Prevention and Treatment of Chemotherapy-induced or Radiotherapy-induced Oral Mucositis. ASTRO 2012 Study Registry Abstract, May 2012.
- 45.Brock J, Morris C, Hotze K, Pikkula B. Supersaturated Calcium Phosphate Rinse vs. Standard of Care for Mitigating Mucositis in Head and Neck Chemoradiation. J Cancer Ther 2018;9:262-7. https://doi.org/10.4236/jct.2018.93023.
- 46.Mohammadi F, Oshvandi K, Kamallan SR et al. Effectiveness of sodium bicarbonate and zinc chloride mouthwashes in the treatment of oral mucositis and quality of life in patients with cancer under chemotherapy. Nurs Open 2022;9(3):1602-11. doi: 10.1002/nop2.1168.
- 47.Oshvandi K, Vafaei SY, Kamallan SR et al. Effectiveness of zinc chloride mouthwashes on oral mucositis and weight of patients with cancer undergoing chemotherapy. BMC Oral Health 2021;21(1):364. doi: 10.1186/s12903-021-01706-w.
- 48.Yarom N, Hovan A, Bossi P et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: vitamins, minerals, and nutritional supplements. Support Care Cancer 2019;27(10):3997-4010.
- 49.Correa MEP, Cheng KKF, Chiang K et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020;28(5):2449-56. doi: 10.1007/s00520-019-05217-x.
- 50.Logan RM, Al-Azri AR, Bossi P et al; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020;28(5):2485-98. doi: 10.1007/s00520-019-05170-9.
- 51.Riley P, Glenny AM, Worthington HV et al. Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors. Cochrane Database Syst Rev 2017;11(11):CD011990. doi: 10.1002/14651858.CD011990.pub2.