Periodontitis and Rheumatoid Arthritis: Current Knowledge and Potential Mechanisms.
Oral-systemic relationships exist for chronic periodontitis (CP) and several systemic diseases, including a bidirectional relationship for CP and diabetes mellitus.1Sanz M, Ceriello A, Buysschaert M et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol 2018;45(2):138-49. doi: 10.1111/jcpe.12808. In addition, systemic chronic inflammatory conditions and CP often co-exist in the same individual. CP is multifactorial and prevalent among middle-aged and older adults, with an estimated overall prevalence of 42% in the general population.2Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000 2020;82(1):257-67. doi:10.1111/prd.12323. In Part 1, we discussed potential associations for CP and psoriatic disease.3Collins FM. Only Skin Deep? Psoriasis, psoriatic arthritis and periodontitis. https://www.colgateoralhealthnetwork.com/article/only-skin-deep-psoriasis-psoriatic-arthritis-and-periodontitis/. In this article, we will provide an overview of the potential mechanisms and relationship for CP and rheumatoid arthritis (RA).
Characteristics of RA
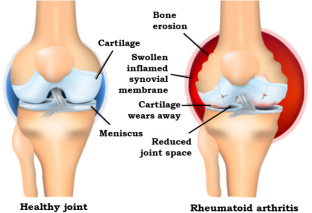
RA is a chronic inflammatory, multifactorial auto-immune disease involving a dysregulated immune response, with a prevalence of up to 1% in the general population.4Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci 2019;20(14):3394. doi:10.3390/ijms20143394.,5Jung G-U, Han J-Y, Hwang K-J et al. Effects of Conventional Synthetic Disease-Modifying Antirheumatic Drugs on Response to Periodontal Treatment in Patients with Rheumatoid Arthritis. BioMed Res Int 2018:Article ID 1465402. https://doi.org/10.1155/2018/1465402.,6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698. Significantly more women than men are affected and risk for onset in adults increases with age.4Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci 2019;20(14):3394. doi:10.3390/ijms20143394. Synovitis (inflammation of the synovium, or capsule, around the joint) and progressive destruction of joints occurs.7Rheumatoid arthritis support network. https://www.rheumatoidarthritis.org/ra/. (Figure 1) In addition to pain and stiffness, patients experience fatigue and in advanced stages loss of motion and loss of function. RA onset and progression is determined by host and environmental risk factors.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698.
Risk Factors for RA
Genetics accounts for 50% to 65% of risk for incident RA.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698. Other host risk factors include epigenetics, neuroendocrine disorders, depression, sleep disorders, PTSD, hormonal/reproductive factors (e.g., early menopause), asthma, atopic dermatitis, rhinitis, COPD, and the presence of other autoimmune-mediated diseases.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698. ,7Rheumatoid arthritis support network. https://www.rheumatoidarthritis.org/ra/. ,8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139. Environmental risk factors include smoking, exposure to airborne contaminants such as silica/textile dusts, the presence of certain microorganisms, poor dietary habits, and inadequate physical exercise.8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139. In addition, CP is a recognized risk factor for RA.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698. ,9Zhou N, Zou F, Cheng X et al. Porphyromonas gingivalis induces periodontitis, causes immune imbalance, and promotes rheumatoid arthritis. doi: 10.1002/JLB.3MA0121-045R. (Table 1)
| Table 1. Risk Factors for RA | |
|---|---|
| Host Factors | Environmental Factors |
| Genetics | Smoking |
| Epigenetics | Exposure to airborne contaminants |
| Neuroendocrine disorders | Poor dietary habits |
| Hormonal/reproductive factors | Inadequate physical exercise |
| Chronic periodontitis | Presence of certain microorganisms |
| Depression, sleep disorders, PTSD | |
| Atopic dermatitis, asthma, rhinitis | |
| COPD | |
| Other autoimmune-mediated diseases | |
Altered Immune Response
The IL-23/IL-17 pathway can alter the innate immune response.10Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015;69:142-59. doi 10.1111/prd.12083.,11Feng Y, Chen Z, Tu SQ et al. Role of Interleukin-17A in the Pathomechanisms of Periodontitis and Related Systemic Chronic Inflammatory Diseases. Front Immunol 2022;13:862415. doi: 10.3389/fimmu.2022.862415. This pathway involves the secretion of IL-17 and IL-22 by T helper 17 cells (Th17), a subset of CD4 T cells. Th17 is increased in amount by IL-23, while IL-17 mediates the innate immune response and also acts as a pro-inflammatory cytokine.10Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015;69:142-59. doi 10.1111/prd.12083.,11Feng Y, Chen Z, Tu SQ et al. Role of Interleukin-17A in the Pathomechanisms of Periodontitis and Related Systemic Chronic Inflammatory Diseases. Front Immunol 2022;13:862415. doi: 10.3389/fimmu.2022.862415.,12Malakouti M, Brown GE, Wang E et al. The role of IL-17 in psoriasis. J Dermatolog Treat 2015;26(1):41-4. doi: 0.3109/09546634.2013.879093. The inflammatory cascade mediated by IL-17 results in increases in other pro-inflammatory cytokines and chemokines, as is observed in synovitis.4Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci 2019;20(14):3394. doi:10.3390/ijms20143394. Ultimately, joint destruction occurs, with bone loss due to osteoclastic activity and collagen degradation by matrix metalloproteinases as well as other cytokines, and cartilage destruction caused by cytokines produced by chondrocytes.
Role of citrullination
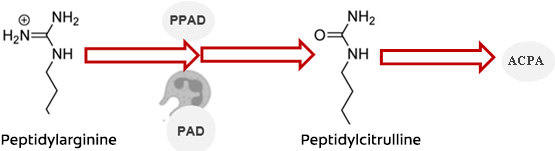
Citrullination occurs physiologically and in disease. It involves the degradation of host peptidylarginine, catalyzed by the enzymes peptidylarginine deiminases (PADs). This results in the production of peptidocitrulline, a citrullinated protein.13Ciesielski O, Biesiekierska M, Panthu B et al. Citrullination in the pathology of inflammatory and autoimmune disorders: recent advances and future perspectives. Cell Mol Life Sci 2022;79(2):94. doi: 10.1007/s00018-022-04126-3. However, dysregulation causes levels of PADs to increase. The resulting increased level of peptidocitrulline acts as an antigen, triggering an autoimmune-mediated response and production of autoantibodies against citrullinated proteins (ACPA).8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139. Increased levels of ACPA, and another autoantibody rheumatoid factor (RF), are detectable prior to the onset of clinically detectable RA, typically by several years.14Deane KD, Holers VM. Rheumatoid Arthritis Pathogenesis, Prediction, and Prevention: An Emerging Paradigm Shift. Arthritis Rheumatol 2021;73(2):181-93. doi: 10.1002/art.41417.,15Vitkov L, Hannig M, Minnich B, Herrmann M. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity 2018;51(6):304-9. doi: 10.1080/08916934.2018.1527907. ,16Lopez-Oliva I, Paropkari AD, Saraswat S et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2018;70(7):1008-13. doi: 10.1002/art.40485. They are also a feature of clinical disease, with greater innate and adaptive responses. (Figure 2)
Similarities between RA and CP
RA and CP share similarities. Both are multifactorial inflammatory conditions with altered innate and adaptive immune responses. Shared characteristics include the IL-23/IL-17 pathway and inflammatory cascade involving cytokines and chemokines, destruction of hard and soft tissues, and a specific genetic trait (human leukocyte antigen – HLA-DRB1).4Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci 2019;20(14):3394. doi:10.3390/ijms20143394.,11Feng Y, Chen Z, Tu SQ et al. Role of Interleukin-17A in the Pathomechanisms of Periodontitis and Related Systemic Chronic Inflammatory Diseases. Front Immunol 2022;13:862415. doi: 10.3389/fimmu.2022.862415.,16Lopez-Oliva I, Paropkari AD, Saraswat S et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2018;70(7):1008-13. doi: 10.1002/art.40485.,17Ancuta C, Iordache C, Ancuta E, Mihailov C. Rheumatoid arthritis and periodontal disease: A complex interplay. Ch 6, pp101-20. http://dx.doi.org/10.5772/65863. ,18Ceccarelli F, Saccucci M, Di Carlo G et al. Periodontitis and Rheumatoid Arthritis: The Same Inflammatory Mediators? Mediators Inflamm 2019;2:6034546. doi: 10.1155/2019/6034546.,19Li R, Tian C, Postlethwaite A et al. Rheumatoid arthritis and periodontal disease: What are the similarities and differences? Int J Rheum Dis 2017;20(12):1887-1901. doi: 10.1111/1756-185X.13240. ,20Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin. Periodontal 2019;46:6-11. doi: 10.1111/jcpe.13046. Genetic variations in pro-inflammatory cytokines common to CP and RA have also been found.21Schulz S, Pütz N, Jurianz E et al. Are There Any Common Genetic Risk Markers for Rheumatoid Arthritis and Periodontal Diseases? A Case-Control Study. Mediators Inflamm 2019;2019:2907062. doi: 10.1155/2019/2907062. However, results reported for genetic factors other than HLA-DRB1 are in conflict.18Ceccarelli F, Saccucci M, Di Carlo G et al. Periodontitis and Rheumatoid Arthritis: The Same Inflammatory Mediators? Mediators Inflamm 2019;2:6034546. doi: 10.1155/2019/6034546. Other shared risk factors include epigenetics, the presence of auto-immune disorders, and smoking which also has a dose-response relationship.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698. ,22Bhat M, Roberts-Thomson K, Do LG. Clustering of risk indicators for periodontal disease: A population-based study. Community Dent Health 2015;32(3):158-62. Up to a quarter of the overall risk for RA is believed to be attributable to smoking, while a 4-fold risk of CP among current smokers has been found.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698. ,23Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: Findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol 2000;71(5):743-51.
CP results from oral dysbiosis, with a shift in the balance of oral microorganisms. The periodontopathogens Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa) are present in high levels in patients with CP and suggested to play a role in the etiology and pathogenesis of RA.16Lopez-Oliva I, Paropkari AD, Saraswat S et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2018;70(7):1008-13. doi: 10.1002/art.40485. In addition, higher salivary levels of IL-17 are found in patients with CP and patients with RA; IL-17 is believed to induce oral dysbiosis.24Martínez-García M, Hernández-Lemus E. Periodontal Inflammation and Systemic Diseases: An Overview. Front Physiol 2021;12:709438. doi: 10.3389/fphys.2021.709438. Furthermore, increased clinical attachment loss in patients with CP is influenced by the involvement of IL-22, which increases synovitis.
Involvement of periodontal pathogens
Pg and Aa play a role in synovitis. Pg expresses a specific PAD (PPAD), while Aa produces leukotoxin-A (LtxA) that triggers PAD in neutrophils, cell death and citrullination.15Vitkov L, Hannig M, Minnich B, Herrmann M. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity 2018;51(6):304-9. doi: 10.1080/08916934.2018.1527907. ,25Konig MF, Abusleme L, Reinholdt J et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921.,26Looh SC, Soo ZMP, Wong JJ et al. Aggregatibacter actinomycetemcomitans as the Aetiological Cause of Rheumatoid Arthritis: What Are the Unsolved Puzzles? Toxins (Basel) 2022;14(1):50. doi: 10.3390/toxins14010050. ,27Gómez-Bañuelos E, Mukherjee A, Darrah E, Andrade F. Rheumatoid Arthritis-Associated Mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Clin Med 2019;8(9):1309. doi: 10.3390/jcm8091309. In both cases, ACPA is produced. Citrullination in CP also involves both PPAD and PAD4 (via neutrophils). In addition, citrullination results in a shift favoring anaerobic bacteria through the generation of ammonia.17Ancuta C, Iordache C, Ancuta E, Mihailov C. Rheumatoid arthritis and periodontal disease: A complex interplay. Ch 6, pp101-20. http://dx.doi.org/10.5772/65863. DNA of Pg, Prevotella nigrescens, Tannerella forsythensis (Tf), T. denticola, F. nucleatum and Prevotella intermedia (Pi) has been found in the synovial fluid of patients with RA.24Martínez-García M, Hernández-Lemus E. Periodontal Inflammation and Systemic Diseases: An Overview. Front Physiol 2021;12:709438. doi: 10.3389/fphys.2021.709438. In another study, antibody titers against Tf, Pi and Pg were higher in the serum and the synovial fluids of patients with RA.24Martínez-García M, Hernández-Lemus E. Periodontal Inflammation and Systemic Diseases: An Overview. Front Physiol 2021;12:709438. doi: 10.3389/fphys.2021.709438.,28Moen K, Brun JG, Valen M et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exper Rheumatol 2006;24:656-63. Additionally, an increased antibody titer against Pg has been observed before RA onset.29Johansson L, Sherina N, Kharlamova N et al. Concentration of antibodies against porphyromonas gingivalis is increased before the onset of symptoms of rheumatoid arthritis. Arthritis Res Ther 2016;18:201. doi: 10.1186/s13075-016-1100-4.
Conversely, in a recent study higher levels of gram-negative anaerobes were found in periodontally healthy patients with RA, but Pg and Aa were not dominant.13Ciesielski O, Biesiekierska M, Panthu B et al. Citrullination in the pathology of inflammatory and autoimmune disorders: recent advances and future perspectives. Cell Mol Life Sci 2022;79(2):94. doi: 10.1007/s00018-022-04126-3. Rather, Cryptobacterium curtum (Cc) was identified in the subgingival microbiome at a level a hundred times higher than that of individuals without RA. Cc also has the ability to produce citrulline and therefore to elicit production of autoantibodies. It was concluded that Cc and other gram-negative anaerobes may contribute to production of autoantibodies and thereby foster the onset of RA, while Pg and Aa may influence disease state.16Lopez-Oliva I, Paropkari AD, Saraswat S et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2018;70(7):1008-13. doi: 10.1002/art.40485. These findings are supported by other studies in which the prevalence of Pg in subgingival biofilm was similar for patients with RA with and without CP, and in which pathogens other than Pg, Aa and Cc were most prolific among patients with RA.8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139.
Study results for RA and CP
In a systematic review and meta-analysis with 17 studies comparing patients with and without RA, a significant association was found between RA and CP.30Fuggle NR, Smith TO, Kaul A, Sofat N. Hand to mouth: a systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front Immunol 2016;7:80. doi: 10.3389/fimmu.2016.00080. In a nationwide, population-based, case–control study a relationship was found for CP and incident RA after adjusting for confounders.31Chen H-H, Huang N, Chen Y-M et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: a nationwide, population-based, case–control study. Ann Rheum Dis 2013;72:1206-11. doi: 10.1136/annrheumdis-2012-201593. However, smoking status was not considered. Similarly, studies have found increased risk for CP and CP of greater severity among patients with RA.8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139.,32Scher JU, Ubeda C, Equinda M et al. Periodontal disease and the oral microbiota in new onset rheumatoid arthritis. Arthritis Rheum 2012; 64:3083-94.,33Berthelot J-M, Le Goff B. Rheumatoid arthritis and periodontal disease. Joint Bone Spine 2010;77(6):537-41.
In contrast, a recent study did not show a relationship for prevalence of CP and RA disease activity.34Gough MRC, Kirkham BW. The prevalence and effect of periodontitis on rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis disease activity: a cross-sectional observational study. Rheumatol 2018;57(Suppl_3). doi.org/10.1093/rheumatology/key075.413. It was noted that this may have been due to underreporting of CP and what was considered the likely small effect of CP.34Gough MRC, Kirkham BW. The prevalence and effect of periodontitis on rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis disease activity: a cross-sectional observational study. Rheumatol 2018;57(Suppl_3). doi.org/10.1093/rheumatology/key075.413. In a cross-sectional study in patients over age 69 with RA, no clear relationship was observed for RA inflammatory markers in patients with CP.35Söderlin MK, Persson GR, Renvert S, Berglund SJ. Cytokines in gingival crevicular fluid in elderly rheumatoid arthritis patients in a population‐based cross‐sectional study: RANTES was associated with periodontitis. J Periodont Res 2021;56(5):907-16. doi: 10.1111/jre.12887. Of note, larger studies have not demonstrated an association for CP and onset of RA, or only a weak one, while major confounders were not considered.6Romão VC, Fonseca JE. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front Med 2021;8:689698. doi:10.3389/fmed.2021.689698.
Potential impact of RA treatment on CP
Studies have conducted on whether improvements in CP are obtained following treatment for RA and vice versa. Therapeutic options for RA treatment include non-steroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying anti-rheumatic drugs which includes methotrexate, hydroxychloroquine, sulfasalazine and biologics.5Jung G-U, Han J-Y, Hwang K-J et al. Effects of Conventional Synthetic Disease-Modifying Antirheumatic Drugs on Response to Periodontal Treatment in Patients with Rheumatoid Arthritis. BioMed Res Int 2018:Article ID 1465402. https://doi.org/10.1155/2018/1465402.,17Ancuta C, Iordache C, Ancuta E, Mihailov C. Rheumatoid arthritis and periodontal disease: A complex interplay. Ch 6, pp101-20. http://dx.doi.org/10.5772/65863. In a recent systematic review of 14 studies with almost five hundred patients and meta-analysis of 4 of these studies, patients with CP taking medications to treat RAs were evaluated for periodontal outcomes.36Zhang J, Xu C, Gao L et al. Influence of anti-rheumatic agents on the periodontal condition of patients with rheumatoid arthritis and periodontitis: A systematic review and meta-analysis. J Periodontal Res 2021;56(6):1099-115. doi: 10.1111/jre.12925. Reductions were found for probing depth, clinical attachment loss, and gingivitis, in patients taking these medications compared to patients with CP but not RA.
Potential impact of non-surgical periodontal therapy
In a recent meta-analysis of 9 studies, the efficacy of non-surgical periodontal treatment in improving RA disease activity was evaluated.37Sun J, Zheng Y, Bian X et al. Non-surgical periodontal treatment improves rheumatoid arthritis disease activity: a meta-analysis. Clin Oral Investig 2021;25(8):4975-85. doi: 10.1007/s00784-021-03807-w. These studies compared RA parameters for patients with both RA and CP who did/did not receive periodontal therapy. Periodontal therapy resulted in statistically significant reductions in the tender joint and swollen joint counts, C-reactive protein levels and RA disease activity scores. In addition, the levels of morning stiffness, erythrocyte sedimentation rate, TNF-α, IL-6 and RF decreased. The researchers concluded that periodontal therapy is effective in reducing disease activity and needed for patients with RA.
In one study, levels of gingival crevicular fluid, IL-6, prostaglandin E2, and matrix metalloproteinase-8 were higher at baseline in patients with RA compared to those without RA.38Kurgan Ş, Fentoğlu Ö, Önder C et al. The effects of periodontal therapy on gingival crevicular fluid matrix metalloproteinase-8, interleukin-6 and prostaglandin E2 levels in patients with rheumatoid arthritis. J Periodontal Res 2016;51(5):586-95. doi:10.1111/jre.12337. Following non-surgical periodontal treatment, the amount of gingival crevicular fluid and level of pro-inflammatory mediators decreased in patients with and without RA three months post-treatment. It was concluded that non-surgical periodontal therapy may be beneficial in controlling local inflammation in patients with RA.
Current and Future Directions
Research continues to be conducted on the nature of the relationship between CP and RA, biomarkers and treatment options for RA. Multi-biomarker tests have generally been found to be more precise than solo biomarkers for RA and to better track disease activity.39Oderda GM, Lawless GC, Wright GC et al. The potential impact of monitoring disease activity biomarkers on rheumatoid arthritis outcomes and costs. Per Med 2018;15(4):291-301. https://doi.org/10.2217/pme-2018-0001. Biomarkers researched include several proteins, numerous inflammatory mediators, microflora, ACPA and RF.8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139.,40Eastman PS, Manning WC, Qureshi F et al. Characterization of a multiplex, 12-biomarker test for rheumatoid arthritis. J Pharm Biomed Anal 2012;70:415-24. In a recent study, serum levels of a protein (14-3-3η) correlated with RA progression and severity, with greater sensitivity in early RA than for RF and ACPA, suggesting greater utility for early screening and detection.41Alashkar DS, Elkhouly RM, Elnaby AYA, Nada DW. Will 14-3-3η Be a New Diagnostic and Prognostic Biomarker in Rheumatoid Arthritis? A Prospective Study of Its Utility in Early Diagnosis and Response to Treatment. Autoimmune Dis 2022;Article ID 1497748. https://doi.org/10.1155/2022/1497748. Current research into RA treatment modalities includes the use of stem cells, particularly early in the disease process, and growth factors.42Martu M-A, Maftei G-A, Luchian I et al. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review. Pharmaceuticals 2021;14:1209. https://doi.org/10.3390/ph14121209. In addition, gene therapy using plasmids is being investigated.42Martu M-A, Maftei G-A, Luchian I et al. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review. Pharmaceuticals 2021;14:1209. https://doi.org/10.3390/ph14121209.
Conclusions
Further studies are needed to elucidate the proposed mechanisms and the relationship between CP and RA.8Eriksson K, Lundmark A, Delgado LF et al. Salivary Microbiota and Host Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front Cell Infect Microbiol 2022;12:841139. doi: 10.3389/fcimb.2022.841139.,16Lopez-Oliva I, Paropkari AD, Saraswat S et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2018;70(7):1008-13. doi: 10.1002/art.40485.,17Ancuta C, Iordache C, Ancuta E, Mihailov C. Rheumatoid arthritis and periodontal disease: A complex interplay. Ch 6, pp101-20. http://dx.doi.org/10.5772/65863. ,20Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin. Periodontal 2019;46:6-11. doi: 10.1111/jcpe.13046. This would also enable more robust understanding and thereby more interprofessional collaboration for early screening, prevention, and treatment.4Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci 2019;20(14):3394. doi:10.3390/ijms20143394. Research into robust biomarkers aids early intervention and novel therapies under investigation are promising for the early management of RA. In the case of gene therapy, this would provide the ability to intervene preventively. In the meantime, existing knowledge supports screening, periodontal therapy as a modality to not only improve periodontal status but also to potentially reduce RA disease activity. Dental professionals can educate patients on the potential associations between CP and RA, and oral care. In addition, stressing the need for tobacco cessation is important since smoking tobacco is a strong modifiable risk factor for both CP and RA.
References
- 1.Dominy SS, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333 https://www.ncbi.nlm.nih.gov/pubmed/30746447.
- 2.Sadrameli M, et al. Linking mechanisms of periodontitis to Alzheimer’s disease. Curr Opin Neurol. 2020;33(2):230-8 https://www.ncbi.nlm.nih.gov/pubmed/32097126.
- 3.Borsa L, et al. Analysis the link between periodontal diseases and Alzheimer’s disease: A systematic review. Int J Environ Res Public Health. 2021;18(17) https://www.ncbi.nlm.nih.gov/pubmed/34501899.
- 4.Costa MJF, et al. Relationship of Porphyromonas gingivalis and Alzheimer’s disease: A systematic review of pre-clinical studies. Clin Oral Investig. 2021;25(3):797-806 https://www.ncbi.nlm.nih.gov/pubmed/33469718.
- 5.Munoz Fernandez SS, Lima Ribeiro SM. Nutrition and Alzheimer disease. Clin Geriatr Med. 2018;34(4):677-97 https://www.ncbi.nlm.nih.gov/pubmed/30336995.
- 6.Aquilani R, et al. Is the Brain Undernourished in Alzheimer’s Disease? Nutrients. 2022;14(9) https://www.ncbi.nlm.nih.gov/pubmed/35565839.
- 7.Fukushima-Nakayama Y, et al. Reduced mastication impairs memory function. J Dent Res. 2017;96(9):1058-66 https://www.ncbi.nlm.nih.gov/pubmed/28621563.
- 8.Kim HB, et al. Abeta accumulation in vmo contributes to masticatory dysfunction in 5XFAD Mice. J Dent Res. 2021;100(9):960-7 https://www.ncbi.nlm.nih.gov/pubmed/33719684.
- 9.Miura H, et al. Relationship between cognitive function and mastication in elderly females. J Oral Rehabil. 2003;30(8):808-11 https://www.ncbi.nlm.nih.gov/pubmed/12880404.
- 10.Lexomboon D, et al. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60(10):1951-6 https://www.ncbi.nlm.nih.gov/pubmed/23035667.
- 11.Elsig F, et al. Tooth loss, chewing efficiency and cognitive impairment in geriatric patients. Gerodontology. 2015;32(2):149-56 https://www.ncbi.nlm.nih.gov/pubmed/24128078.
- 12.Kim EK, et al. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch Gerontol Geriatr. 2017;70:209-13 https://www.ncbi.nlm.nih.gov/pubmed/28214402.
- 13.Kim MS, et al. The association between mastication and mild cognitive impairment in Korean adults. Medicine (Baltimore). 2020;99(23):e20653 https://www.ncbi.nlm.nih.gov/pubmed/32502052.
- 14.Cardoso MG, et al. Relationship between functional masticatory units and cognitive impairment in elderly persons. J Oral Rehabil. 2019;46(5):417-23 https://www.ncbi.nlm.nih.gov/pubmed/30614023.
- 15.Popovac A, et al. Oral health status and nutritional habits as predictors for developing alzheimer’s disease. Med Princ Pract. 2021;30(5):448-54 https://www.ncbi.nlm.nih.gov/pubmed/34348313.
- 16.Park T, et al. More teeth and posterior balanced occlusion are a key determinant for cognitive function in the elderly. Int J Environ Res Public Health. 2021;18(4) https://www.ncbi.nlm.nih.gov/pubmed/33669490.
- 17.Lin CS, et al. Association between tooth loss and gray matter volume in cognitive impairment. Brain Imaging Behav. 2020;14(2):396-407 https://www.ncbi.nlm.nih.gov/pubmed/32170642.
- 18.Kumar S, et al. Oral health status and treatment need in geriatric patients with different degrees of cognitive impairment and dementia: a cross-sectional study. J Family Med Prim Care. 2021;10(6):2171-6 https://www.ncbi.nlm.nih.gov/pubmed/34322409.
- 19.Delwel S, et al. Chewing efficiency, global cognitive functioning, and dentition: A cross-sectional observational study in older people with mild cognitive impairment or mild to moderate dementia. Front Aging Neurosci. 2020;12:225 https://www.ncbi.nlm.nih.gov/pubmed/33033478.
- 20.Da Silva JD, et al. Association between cognitive health and masticatory conditions: a descriptive study of the national database of the universal healthcare system in Japan. Aging (Albany NY). 2021;13(6):7943-52 https://www.ncbi.nlm.nih.gov/pubmed/33739304.
- 21.Galindo-Moreno P, et al. The impact of tooth loss on cognitive function. Clin Oral Investig. 2022;26(4):3493-500 https://www.ncbi.nlm.nih.gov/pubmed/34881401.
- 22.Stewart R, et al. Adverse oral health and cognitive decline: The health, aging and body composition study. J Am Geriatr Soc. 2013;61(2):177-84 https://www.ncbi.nlm.nih.gov/pubmed/23405916.
- 23.Dintica CS, et al. The relation of poor mastication with cognition and dementia risk: A population-based longitudinal study. Aging (Albany NY). 2020;12(9):8536-48 https://www.ncbi.nlm.nih.gov/pubmed/32353829.
- 24.Kim MS, Han DH. Does reduced chewing ability efficiency influence cognitive function? Results of a 10-year national cohort study. Medicine (Baltimore). 2022;101(25):e29270 https://www.ncbi.nlm.nih.gov/pubmed/35758356.
- 25.Ko KA, et al. The Impact of Masticatory Function on Cognitive Impairment in Older Patients: A Population-Based Matched Case-Control Study. Yonsei Med J. 2022;63(8):783-9 https://www.ncbi.nlm.nih.gov/pubmed/35914761.
- 26.Garre-Olmo J. [Epidemiology of Alzheimer’s disease and other dementias]. Rev Neurol. 2018;66(11):377-86 https://www.ncbi.nlm.nih.gov/pubmed/29790571.
- 27.Stephan BCM, et al. Secular Trends in Dementia Prevalence and Incidence Worldwide: A Systematic Review. J Alzheimers Dis. 2018;66(2):653-80 https://www.ncbi.nlm.nih.gov/pubmed/30347617.
- 28.Lopez OL, Kuller LH. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer’s disease and other dementias. Handb Clin Neurol. 2019;167:139-48 https://www.ncbi.nlm.nih.gov/pubmed/31753130.
- 29.Ono Y, et al. Occlusion and brain function: mastication as a prevention of cognitive dysfunction. J Oral Rehabil. 2010;37(8):624-40 https://www.ncbi.nlm.nih.gov/pubmed/20236235.
- 30.Kubo KY, et al. Masticatory function and cognitive function. Okajimas Folia Anat Jpn. 2010;87(3):135-40 https://www.ncbi.nlm.nih.gov/pubmed/21174943.
- 31.Chen H, et al. Chewing Maintains Hippocampus-Dependent Cognitive Function. Int J Med Sci. 2015;12(6):502-9 https://www.ncbi.nlm.nih.gov/pubmed/26078711.
- 32.Azuma K, et al. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int J Mol Sci. 2017;18(8) https://www.ncbi.nlm.nih.gov/pubmed/28771175.
- 33.Chuhuaicura P, et al. Mastication as a protective factor of the cognitive decline in adults: A qualitative systematic review. Int Dent J. 2019;69(5):334-40 https://www.ncbi.nlm.nih.gov/pubmed/31140598.
- 34.Lopez-Chaichio L, et al. Oral health and healthy chewing for healthy cognitive ageing: A comprehensive narrative review. Gerodontology. 2021;38(2):126-35 https://www.ncbi.nlm.nih.gov/pubmed/33179281.
- 35.Tada A, Miura H. Association between mastication and cognitive status: A systematic review. Arch Gerontol Geriatr. 2017;70:44-53 https://www.ncbi.nlm.nih.gov/pubmed/28042986.
- 36.Ahmed SE, et al. Influence of Dental Prostheses on Cognitive Functioning in Elderly Population: A Systematic Review. J Pharm Bioallied Sci. 2021;13(Suppl 1):S788-S94 https://www.ncbi.nlm.nih.gov/pubmed/34447202.
- 37.Tonsekar PP, et al. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology. 2017;34(2):151-63 https://www.ncbi.nlm.nih.gov/pubmed/28168759.
- 38.Nangle MR, Manchery N. Can chronic oral inflammation and masticatory dysfunction contribute to cognitive impairment? Curr Opin Psychiatry. 2020;33(2):156-62 https://www.ncbi.nlm.nih.gov/pubmed/31895157.
- 39.Nakamura T, et al. Oral dysfunctions and cognitive impairment/dementia. J Neurosci Res. 2021;99(2):518-28 https://www.ncbi.nlm.nih.gov/pubmed/33164225.
- 40.Weijenberg RAF, et al. Mind your teeth-The relationship between mastication and cognition. Gerodontology. 2019;36(1):2-7 https://www.ncbi.nlm.nih.gov/pubmed/30480331.
- 41.Asher S, et al. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J Am Geriatr Soc. 2022;70(9):2695-709 https://www.ncbi.nlm.nih.gov/pubmed/36073186.
- 42.Lin CS. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr. 2018;18(1):5 https://www.ncbi.nlm.nih.gov/pubmed/29304748.
- 43.Wu YT, et al. The changing prevalence and incidence of dementia over time – current evidence. Nat Rev Neurol. 2017;13(6):327-39 https://www.ncbi.nlm.nih.gov/pubmed/28497805.
- 44.Desai M, Park T. Deprescribing practices in Canada: A scoping review. Can Pharm J (Ott) 2022;155(5):249-57. doi: 10.1177/17151635221114114.
- 45.Kakkar M, Barmak AB, Arany S. Anticholinergic medication and dental caries status in middle-aged xerostomia patients-a retrospective study. J Dent Sci 2022;17(3):1206-11. doi: 10.1016/j.jds.2021.12.014.
- 46.Kaur M, Himadi E, Chi DL. Prevalence of xerostomia in an adolescent inpatient psychiatric clinic: a preliminary study. Special Care Dent 2016;36:60-5.
- 47.Okamoto A, Miyachi H, Tanaka K et al. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther 2016;41(6):684-8. doi: 10.1111/jcpt.12449.
- 48.Centers for Disease Control and Prevention. Autism Spectrum Disorder (ASD). Data & Statistics on Autism Spectrum Disorder. https://www.cdc.gov/ncbddd/autism/data.html.
- 49.Loo CY, Graham RM, Hughes CV. The caries experience and behavior of dental patients with autism spectrum disorder. J Am Dent Assoc 2008;139(11):1518-24.
- 50.Allison KH, Hammond MEH, Dowsett M et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 2020 38:12, 1346-66. https://ascopubs.org/doi/10.1200/JCO.19.02309.
- 51.Feinstein JA, Feudtner C, Kempe A, Orth LE. Anticholinergic Medications and Parent-Reported Anticholinergic Symptoms in Neurologically Impaired Children. J Pain Symptom Manage 2022:S0885-3924(22)00952-6. doi: 10.1016/j.jpainsymman.2022.10.013.
- 52.Ikram M, Shaikh NF, Sambamoorthi U. A Linear Decomposition Approach to Explain Excess Direct Healthcare Expenditures Associated with Pain Among Adults with Osteoarthritis. Health Serv Insights 2022;15:11786329221133957. doi: 10.1177/11786329221133957.
- 53.Bevilacqua KG, Brinkley C, McGowan J et al. “We are Getting Those Old People Things.” Polypharmacy Management and Medication Adherence Among Adult HIV Patients with Multiple Comorbidities: A Qualitative Study. Patient Prefer Adherence 2022;16:2773-80. doi: 10.2147/PPA.S382005.
- 54.Chertcoff A, Ng HS, Zhu F et al. Polypharmacy and multiple sclerosis: A population-based study. Mult Scler 2022:13524585221122207. doi: 10.1177/13524585221122207.
- 55.Aguglia A, Natale A, Fusar-Poli L et al. Complex polypharmacy in bipolar disorder: Results from a real-world inpatient psychiatric unit. Psychiatry Res 2022;318:114927. doi: 10.1016/j.psychres.2022.114927.
- 56.Cheng JJ, Azizoddin AM, Maranzano MJ et al. Polypharmacy in Oncology. Clin Geriatr Med 2022;38(4):705-14. doi: 10.1016/j.cger.2022.05.010.
- 57.Betts AC, Murphy CC, Shay LA et al. Polypharmacy and medication fill nonadherence in a population-based sample of adolescent and young adult cancer survivors, 2008-2017. J Cancer Surviv 2022 Nov 8. doi: 10.1007/s11764-022-01274-0.
- 58.Macrotrends. U.S. Life Expectancy 1950-2023. https://www.macrotrends.net/countries/USA/united-states/life-expectancy.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2022/07/periodontitis-and-rheumatoid-arthitis-2.jpg)