Salivary Biomarkers and Diagnostics – Part 2: Biomarkers and Oral Disease
Saliva has been described as ‘the defender of the oral cavity’ and provides for the protection of hard and soft tissues; aids taste, swallowing and digestion; and offers antimicrobial properties.1Nieuw Amerongen AV, Veerman EC. Saliva–the defender of the oral cavity. Oral Dis. 2002;8(1):12-22. doi: 10.1034/j.1601-0825.2002.1o816.x. As discussed in Part 1 of this series, saliva contains more than two thousand proteins, enzymes, electrolytes, small organic molecules and antimicrobials.2Podzimek S, Vondrackova L, Duskova J, Janatova T, Broukal Z. Salivary markers for periodontal and general diseases. Disease Markers 2016;Article ID 9179632. https://doi.org/10.1155/2016/9179632. Whole saliva also contains plasma-derived components, sloughed epithelial cells, microorganisms and their associated products, gingival crevicular fluid, debris, and nasopharyngeal discharge.3Genco RJ. Salivary diagnostic tests. J Am Dent Assoc 2012;143(10 suppl):3S-5S. (Figure 1) In the context of oral disease the research, identification and use of salivary biomarkers is ongoing for many conditions. In this article we will discuss some of the salivary biomarkers researched and used for periodontal disease and oral/oropharyngeal cancer.
Periodontal Disease
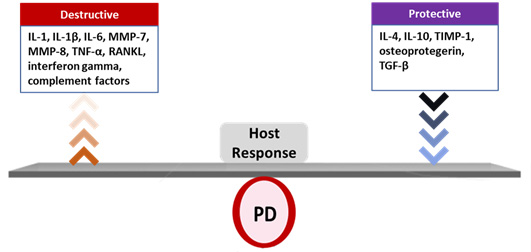
Biofilm-induced periodontal disease (PD) is a polymicrobial, multifactorial inflammatory disease resulting from dysbiosis.4Roberts FA, Darveau RP. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontol 2000 2015;69(1):18-27. Its onset and progression is determined by the host response, together with modifying factors.5Lang NP, Bartold MP. Periodontal health. J Clin Periodontol 2018;45(Suppl 20):S9-16.,6Bartold PM. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontol 2000 2018;78(1):7-11. The host response includes the release of pro-inflammatory mediators, among them interleukin-1 (IL-1), IL-6, tumor necrosis factor alpha, some matrix metalloproteinases (MMPs), complement factors, RANKL and interferon gamma, as well as protective inflammatory mediators such as IL-4, IL-10, TIMP-1, osteoprotegerin and TGF-β.7Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014;64(1):57-80. ,8Liu YC, Lerner UH, Teng YT. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 2010;52:163-206. (Figure 2) Together with other host-derived factors and periodontal pathogens, these mediators have been researched alone and in combination as salivary biomarkers for PD.
Goals for salivary testing include screening, risk assessment/prediction, diagnosis, monitoring and outcomes assessment. In addition to being easier than sampling blood or urine, or taking swabs, salivary testing is noninvasive. It also offers an easier method of collecting samples in comparison to paper point sampling of gingival crevicular fluid.9Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent 2009;22(4):241-8. Further, inherent intra- and inter-operator variability are reported when traditional methods are used to assess PD, including bleeding on probing (BOP), pocket probing depth (PPD), clinical attachment loss (CAL) and radiographic interpretation.10Wang SF, Leknes KN, Zimmerman GJ, Sigurdsson TJ, Wikesjo UM, Selvig KA. Intra- and inter-examiner reproducibility in constant force probing. J Clin Periodontol 1995;22:918-22. doi: 10.1111/j.1600-051X.1995.tb01795.x. ,11Bogren A, Teles R, Torresyap G, Haffajee AD, Socransky SS, Lindhe J, et al. A three-year prospective study of adult subjects with gingivitis. I: clinical periodontal parameters. J Clin Periodontol. 2007; 34 (1):1–6. https://doi.org/10.1111/j.1600-051X.2006.01000.x.,12AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231. These methods measure prior destruction and established disease rather than current activity or risk for disease.10Wang SF, Leknes KN, Zimmerman GJ, Sigurdsson TJ, Wikesjo UM, Selvig KA. Intra- and inter-examiner reproducibility in constant force probing. J Clin Periodontol 1995;22:918-22. doi: 10.1111/j.1600-051X.1995.tb01795.x. ,11Bogren A, Teles R, Torresyap G, Haffajee AD, Socransky SS, Lindhe J, et al. A three-year prospective study of adult subjects with gingivitis. I: clinical periodontal parameters. J Clin Periodontol. 2007; 34 (1):1–6. https://doi.org/10.1111/j.1600-051X.2006.01000.x.,12AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231. Additionally, the sensitivity and positive predictive value of clinical and radiographic measurements and interpretation is low for both periodontitis and peri-implantitis.13Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. doi: 10.1038/nrdp.2017.38.
In 2006, research on salivary biomarkers for PD resulted in the development of a rapid oral rinse assay test that quantified the level of oral neutrophils present.14Bender JS, Thang H, Glogauer M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J Periodontal Res 2006;41(3):214-20. doi: 10.1111/j.1600-0765.2005.00861.x. It was found that the level of neutrophils dropped significantly, with a 43% reduction, following a favorable response to periodontal therapy. No reduction was reported for those who did not respond favorably. Transcriptome research using salivary mRNA has also been used in patients with and without PD to identify gene overexpression/underexpression.15Kim JJ, Kim CJ, Camargo PM. Salivary biomarkers in the diagnosis of periodontal diseases. J Calif Dent Assoc 2013;41(2):119-24. CXCL10 and MMP-7 were found in separate studies to be significantly elevated in patients with CP compared to patients with a healthy periodontium.16Aldahlawi S, Youssef AR, Shahabuddin S. Evaluation of chemokine CXCL10 in human gingival crevicular fluid, saliva, and serum as periodontitis biomarker. J Inflamm Res 2018;11:389-396. doi: 10.2147/JIR.S177188. ,17Lundmark A, Johannsen G, Eriksson K, Kats A, Jansson L, Tervahartiala T, et al. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J Clin Periodontol 2016;44(3):247-54. In the study evaluating MMP-7 it was also found that salivary mucin 4 levels decreased in the patients with periodontitis.17Lundmark A, Johannsen G, Eriksson K, Kats A, Jansson L, Tervahartiala T, et al. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J Clin Periodontol 2016;44(3):247-54. Salivary metabolites generally vary with age of the patient, while the bacterial metabolite phenylacetate was strongly associated with PD status across all age groups.18Liebsch C, Pitchika V, Pink C, Samietz S, Kastenmüller G, Artati A, et al. The Saliva Metabolome in Association to Oral Health Status. J Dent Res 2019;98(6):642-651. doi: 10.1177/0022034519842853. Phenylacetate is considered a promising biomarker for periodontitis.18Liebsch C, Pitchika V, Pink C, Samietz S, Kastenmüller G, Artati A, et al. The Saliva Metabolome in Association to Oral Health Status. J Dent Res 2019;98(6):642-651. doi: 10.1177/0022034519842853.
Combinations of biomarkers in a single test may be more predictive of disease progression/stability than one biomarker.19Korte DL, Kinney J. Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontol 2000 2016;70:26-37. Promising results for PD monitoring have been found for the combination of MMP-8, which is elevated prior to the active phase of PD, osteoprotegerin and macrophage inflammatory protein-1x. Additionally, the combination of MMP-8 and IL1- β demonstrates a positive correlation with BOP, CAL and the percentage of sites with a PPD ≥4 mm.19Korte DL, Kinney J. Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontol 2000 2016;70:26-37. Other research has focused on salivary soluble toll-like receptors (sTLRs) that recognize microorganisms as part of the disease process, their co-receptors, mRNAs and sloughed epithelial cells as salivary biomarkers.12AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231.,20Prakasam S, Srinivasan M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis 2014;20(2):171-7. doi: 10.1111/odi.12085. sTLR-2 and IL-4 have been reported to correlate with the progression of periodontitis.21Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 2016;6(1):66-75. doi:10.1016/j.jobcr.2015.08.006.(Table 1)
| Table 1. PD salivary biomarkers being researched/used |
|---|
| Neutrophils |
| CXCL10 |
| MMP-7 |
| MMP-8 + osteoprotegerin + macrophage inflammatory protein-1x |
| MMP-8 + IL1- β |
| Soluble toll-like receptors (sTLRs) + coreceptors |
| mRNAs |
| IL-4 |
| Other host-derived factors |
| Periodontal pathogens |
| Phenylacetate (bacterial metabolite) |
| Sloughed epithelial cells |
Currently, commercially available chairside salivary tests in the United States and Canada include a 30-second ‘swish and spit’ test where a sample is collected in a test tube, mailed in and analyzed in the laboratory for periodontal pathogens (MyPerioPath, OralDNA Labs).21Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 2016;6(1):66-75. doi:10.1016/j.jobcr.2015.08.006. A second chairside salivary test uses lateral flow technology for a 5-minute point-of-care test (PerioSafe), based on monoclonal antibodies, that assesses the level of active MMP-8.22Alassiri S, Parnanen P, Rathnayake N, Johannsen G, Heikkinen AM, Lazzara R, et al. The ability of quantitative, specific, and sensitive point-of-care/chair-side oral fluid immunotests for aMMP-8 to detect periodontal and peri-implant diseases. Dis Markers 2018:1306396. doi: 10.1155/2018/1306396. This test has been validated in 8 countries, including the United States, and provides information on disease activity based on increased aMMP-8. It has been found to be more sensitive than measurement of standard parameters, to be useful as a predictive tool, for screening and diagnosis, and is considered promising as a biomarker to assess episodic disease progression and the timing of preventive interventions to prevent progression from gingivitis to periodontitis. While unable to predict disease onset, another salivary test identifies periodontal pathogens and their concentrations using DNA-polymerase chain reaction (DNA-PCR).15Kim JJ, Kim CJ, Camargo PM. Salivary biomarkers in the diagnosis of periodontal diseases. J Calif Dent Assoc 2013;41(2):119-24.
Gingivitis
Recently, sTLRs have also been researched as biomarkers for gingivitis.12AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231. Statistically significant reductions in sTLR-4 were found for patients with gingivitis, while sTLR-2 trended upwards. Concentrations of their co-receptor have been found to increase for patients with gingivitis and those with chronic periodontitis. In other research, the concentrations of MMP-8, MMP-9, cystatin C, lactoferrin, myeloperoxidase (MPO), platelet-activating factor, cathepsin B and pyridinoline were determined in patients with and without gingivitis using enzyme-linked immunosorbent assay kits and compared to measurements of standard parameters used for the diagnosis of gingivitis and periodontitis.23Hong I, Pae HC, Song YW, Cha J-K, Lee J-S, Paik J-W, Choi S-H, et al. Oral fluid biomarkers for diagnosing gingivitis in human: A cross-sectional study. J Clin Med 2020;9(6):1720. doi:10.3390/jcm9061720. Only MMP-8 and MPO levels correlated with strongly with gingivitis. It was concluded that these were accurate for the diagnosis of gingivitis but that a more sensitive test was required.
Oral and Oropharyngeal Cancer
The majority of oral cancers are squamous cell carcinomas (OSCC), mainly diagnosed at an advanced stage. Treatment results in considerable morbidity, recurrence frequently occurs, and the overall five-year survival rate is less than 50%.24Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476. ,25Franzmann EJ, Donovan MJ. Effective early detection of oral cancer using a simple and inexpensive point of care device in oral rinses. Expert Rev Mol Diagn 2018;18(10):837-44. doi: 10.1080/14737159.2018.1523008. This makes the promise of routine screening for salivary biomarkers for the detection of pre-malignant and malignant oral lesions particularly interesting. In a recent systematic review of articles published between 1995 and 2017, it was concluded that salivary proteins represented the majority of potential biomarkers.26Kaur J, Jacobs R, Huang Y, Salvo N, Politis C. Salivary biomarkers for oral cancer and pre-cancer screening: a review. Clin Oral Investig 2018;22(2):633-40. doi: 10.1007/s00784-018-2337-x. Numerous biomarkers are being utilized, including circulating tumor DNA (ctDNA), micro RNAs, extracellular vesicles, circulating tumor cells and endothelin receptor type B hypermethylation. More than 100 salivary components have been reported to differ in concentration in patients with and without OSCC.24Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476. ,27Roi A, Rusu LC, Roi CI, Luca RE, Boia S, Munteanu RI. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers 2019:8761860.
In one study assessing seven mRNAs in the saliva of patients with OSCC, smokers with CP, non-smokers with CP, and healthy subjects, only the level of S100 calcium-binding protein P (S100P) was significantly higher for patients with OSCC than for subjects with CP (smokers and non-smokers).28Cheng YL, Jordan L, Chen HS, Kang D, Oxford L, Plemons J, et al. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J Periodontal Res 2017;52(3):428-37. doi: 10.1111/jre.12407. The other biomarkers tested included IL-8, IL-1β, dual specificity phosphatase 1, ornithine decarboxylase antizyme 1, spermidine/spermine N1-acetyltransferase 1, and H3 histone family 3A. CP was concluded to influence the results and therefore reduce the usefulness of the other biomarker candidates. In a Hungarian study, salivary protein S100A9 and IL-6 were determined to be useful protein biomarkers for detecting OSCC.29Csősz É, Lábiscsák P, Kalló G, Márkus B, Emri M, Szabó A, et al. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS One 2017;12(5):e0177282. doi:10.1371/journal.pone.0177282. Additionally, changes in the compositions of intraoral Bacillus, Enterococcus, Parvimonas, Peptostreptococcus, and Slackia genera have been found to correlate with malignant transformation in oral cancer.30Lee WH, Chen HM, Yang SF, Liang C, Peng CY, Lin FM, et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep 2017;7(1):16540. doi: 10.1038/s41598-017-16418-x. It is also believed that salivary cortisol concentrations may be useful for staging tumors.27Roi A, Rusu LC, Roi CI, Luca RE, Boia S, Munteanu RI. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers 2019:8761860.
However, in one review it was stated that accurate detection of early-stage OSCC has not been achieved using a single marker, and it was proposed that a panel of biomarkers could provide sufficient sensitivity for this purpose.21Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 2016;6(1):66-75. doi:10.1016/j.jobcr.2015.08.006. Further, in a systematic review conducted in 2017, it was concluded at that time that the heterogeneity of studies with respect to salivary biomarkers and methodology precluded inter-study comparisons of the levels of biomarkers in patients with and without OSCC.31Stuani VT, Rubira CM, Sant’Ana AC, Santos PS. Salivary biomarkers as tools for oral squamous cell carcinoma diagnosis: A systematic review. Head Neck 2017;39(4):797-811. doi: 10.1002/hed.24650.
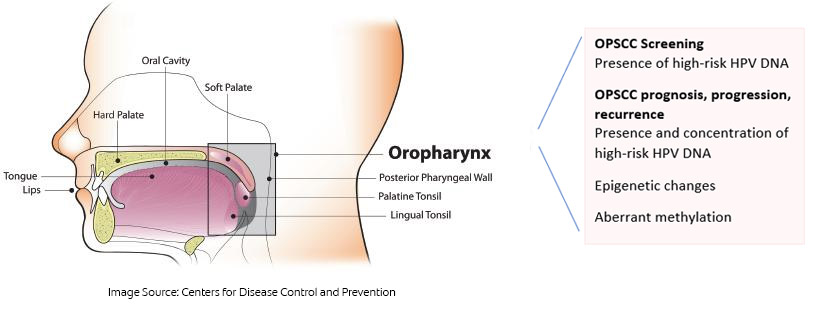
Oropharyngeal squamous cell carcinoma (OPSCC) and HPV
More than 70% of cases of OPSCC in the United States are caused by infection with high-risk human papillomavirus (HPV), primarily HPV-16.32Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107(6):djv086. As such, screening for HPV-16 offers an opportunity to identify individuals at increased risk for OPSCC. Researchers recently reported the early diagnosis of an asymptomatic patient using salivary testing for HPV DNA, with the patient then receiving curative treatment with low morbidity.33Tang KD, Vasani S, Taheri T, Walsh LJ, Hughes BGM, Kenny L, Punyadeera C. An occult HPV-driven oropharyngeal squamous cell carcinoma discovered through a saliva test. Front Oncol 2020;10:408. doi: 10.3389/fonc.2020.00408. A commercially available test can be used to screen for HPV DNA (OraRisk HPV test, OralDNA Labs).34OralDNA. OraRisk HPV. https://www.oraldna.com/test/ohpv-complete/ The patient swishes and gargles with 3 ml of saline and then spits into a collection tube that is sealed and sent to the laboratory for DNA-PCR analysis and genotyping. The test report includes whether or not HPV DNA was detected and, if so, which strains of HPV the DNA belonged to and the risk level for these strains. Dental and medical professionals can then discuss the results with their patients and develop a monitoring plan for earlier identification of disease should the test be positive for high-risk HPV. Patients should also understand that the presence of HPV DNA does not necessarily mean that the virus is active, it means that it is present.
Treatment prognosis and management for OSCC and OPSCC
Salivary biomarkers are being used to monitor treatment and outcomes for OSCC, OPSCC and other head and neck cancers.24Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476. In one study, ctDNA was found in the saliva of all patients with OSCC who later had a recurrence and not in patients with no recurrence, suggesting a role in treatment monitoring.35Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med 2015;7:293ra104. doi: 10.1126/scitranslmed.aaa8507. Increases in salivary concentrations of IL-17A and F, and TNF-α, have been found to be statistically significant for disease progression, salivary miR-139-5p to be useful for evaluation of treatment response and OSCC recurrence, and IL-6 may help in identifying advanced OSCC.24Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476. ,36Zielińska K, Karczmarek-Borowska B, Kwaśniak K, Czarnik-Kwaśniak J, Ludwin A, Lewandowski B, Tabarkiewicz J. Salivary IL-17A, IL-17F, and TNF-α are associated with disease advancement in patients with oral and oropharyngeal cancer. J Immunol Res 2020:3928504. doi: 10.1155/2020/3928504. ,37Lee LT, Wong YK, Hsiao HY, Wang YW, Chan MY, Chang KW. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2018;47(6):699-707. doi: 10.1016/j.ijom.2017.09.016. Furthermore, another biomarker (CTC) can provide predictive information on the prognosis.24Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476. There is also interest in evaluating extracellular vesicles, which act as a transport vehicle for nucleic acids and proteins associated with inhibition or promotion of tumor formation, for diagnostic and prognostic purposes.24Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476.
Salivary testing for HPV-DNA and epigenetic alterations are used to assess treatment outcomes for OPSCC. One retrospective cohort study on salivary testing included patients who had already been treated for HPV-associated OPSCC, those yet to be treated and controls.38Shen S, Saito Y, Ren S, Liu C, Guo T, Qualliotine J, et al. Targeting viral DNA and promoter hypermethylation in salivary rinses for recurrent HPV-positive oropharyngeal cancer. Otolaryngol Head Neck Surg 2020;162(4):512-9. doi: 10.1177/0194599820903031. The presence of high-risk HPV DNA or aberrant methylation was found to be associated with OPSCC and its recurrence. It was suggested that testing for these biomarkers could be used to monitor patients. P16 immunostaining is also used to stage OPSCC. In a study with an observational cohort design, an almost 20-fold level of salivary HPV DNA was present in individuals with advanced recurrent and persistent OPSCC.39Hanna GJ, Lau CJ, Mahmood U, Supplee JG, Mogili AR, Haddad RI, et al. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol 2019;95:120-6. doi: 10.1016/j.oraloncology. (Figure 3) It was concluded that salivary testing for HPV-DNA provided results that were informative for tumor burden and predictive for treatment outcomes in patients with advanced OPSCC.
Conclusions
During the last two decades, rapid developments in the use of salivary biomarkers for oral diseases and conditions has occurred.40Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am 2011;55(1):159-78. doi:10.1016/j.cden.2010.08.004. A need for larger cohorts, comparable study designs, more validation and population group-specific research for different ethnic groups, and more validation for solo and multi-biomarker panels are reported.12AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231.,21Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 2016;6(1):66-75. doi:10.1016/j.jobcr.2015.08.006.,23Hong I, Pae HC, Song YW, Cha J-K, Lee J-S, Paik J-W, Choi S-H, et al. Oral fluid biomarkers for diagnosing gingivitis in human: A cross-sectional study. J Clin Med 2020;9(6):1720. doi:10.3390/jcm9061720.,29Csősz É, Lábiscsák P, Kalló G, Márkus B, Emri M, Szabó A, et al. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS One 2017;12(5):e0177282. doi:10.1371/journal.pone.0177282.,31Stuani VT, Rubira CM, Sant’Ana AC, Santos PS. Salivary biomarkers as tools for oral squamous cell carcinoma diagnosis: A systematic review. Head Neck 2017;39(4):797-811. doi: 10.1002/hed.24650. Accurate and cost-effective mass screening and early diagnosis and treatment, as noted in Part 1, can and could enable improved care, and reduce morbidity and mortality. As the use of salivary biomarkers increases, medical professionals will be able to more easily screen for oral conditions and refer patients to dental professionals, and vice versa. Increased collaboration in screening and treating oral, systemic and oral-systemic conditions will also be possible. At the current time, research into biomarkers is ongoing. Salivary biomarkers are promising as part of continued developments in P4 medicine and dentistry, to deliver personalized treatment plans and care that would enhance outcomes.6Bartold PM. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontol 2000 2018;78(1):7-11.,12AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231.,41Collins FM. Integrating the 4Ps into patient care. Available at: https://www.colgateoralhealthnetwork.com/article/integrating-the-4ps-into-patient-care/. ,42Knight ET, Murray Thomson W. A public health perspective on personalized periodontics. Periodontol 2000. 2018 Oct;78(1):195-200. doi: 10.1111/prd.12228. PMID: 30198135.
References
- 1.Nieuw Amerongen AV, Veerman EC. Saliva–the defender of the oral cavity. Oral Dis. 2002;8(1):12-22. doi: 10.1034/j.1601-0825.2002.1o816.x.
- 2.Podzimek S, Vondrackova L, Duskova J, Janatova T, Broukal Z. Salivary markers for periodontal and general diseases. Disease Markers 2016;Article ID 9179632. https://doi.org/10.1155/2016/9179632.
- 3.Genco RJ. Salivary diagnostic tests. J Am Dent Assoc 2012;143(10 suppl):3S-5S.
- 4.Roberts FA, Darveau RP. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontol 2000 2015;69(1):18-27.
- 5.Lang NP, Bartold MP. Periodontal health. J Clin Periodontol 2018;45(Suppl 20):S9-16.
- 6.Bartold PM. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontol 2000 2018;78(1):7-11.
- 7.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014;64(1):57-80.
- 8.Liu YC, Lerner UH, Teng YT. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 2010;52:163-206.
- 9.Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent 2009;22(4):241-8.
- 10.Wang SF, Leknes KN, Zimmerman GJ, Sigurdsson TJ, Wikesjo UM, Selvig KA. Intra- and inter-examiner reproducibility in constant force probing. J Clin Periodontol 1995;22:918-22. doi: 10.1111/j.1600-051X.1995.tb01795.x.
- 11.Bogren A, Teles R, Torresyap G, Haffajee AD, Socransky SS, Lindhe J, et al. A three-year prospective study of adult subjects with gingivitis. I: clinical periodontal parameters. J Clin Periodontol. 2007; 34 (1):1–6. https://doi.org/10.1111/j.1600-051X.2006.01000.x.
- 12.AlQallaf H, Hamada Y, Blanchard S, Shin D, Gregory R, Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One 2018;13(12):e0200231. doi:10.1371/journal.pone.0200231.
- 13.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. doi: 10.1038/nrdp.2017.38.
- 14.Bender JS, Thang H, Glogauer M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J Periodontal Res 2006;41(3):214-20. doi: 10.1111/j.1600-0765.2005.00861.x.
- 15.Kim JJ, Kim CJ, Camargo PM. Salivary biomarkers in the diagnosis of periodontal diseases. J Calif Dent Assoc 2013;41(2):119-24.
- 16.Aldahlawi S, Youssef AR, Shahabuddin S. Evaluation of chemokine CXCL10 in human gingival crevicular fluid, saliva, and serum as periodontitis biomarker. J Inflamm Res 2018;11:389-396. doi: 10.2147/JIR.S177188.
- 17.Lundmark A, Johannsen G, Eriksson K, Kats A, Jansson L, Tervahartiala T, et al. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J Clin Periodontol 2016;44(3):247-54.
- 18.Liebsch C, Pitchika V, Pink C, Samietz S, Kastenmüller G, Artati A, et al. The Saliva Metabolome in Association to Oral Health Status. J Dent Res 2019;98(6):642-651. doi: 10.1177/0022034519842853.
- 19.Korte DL, Kinney J. Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontol 2000 2016;70:26-37.
- 20.Prakasam S, Srinivasan M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis 2014;20(2):171-7. doi: 10.1111/odi.12085.
- 21.Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 2016;6(1):66-75. doi:10.1016/j.jobcr.2015.08.006.
- 22.Alassiri S, Parnanen P, Rathnayake N, Johannsen G, Heikkinen AM, Lazzara R, et al. The ability of quantitative, specific, and sensitive point-of-care/chair-side oral fluid immunotests for aMMP-8 to detect periodontal and peri-implant diseases. Dis Markers 2018:1306396. doi: 10.1155/2018/1306396.
- 23.Hong I, Pae HC, Song YW, Cha J-K, Lee J-S, Paik J-W, Choi S-H, et al. Oral fluid biomarkers for diagnosing gingivitis in human: A cross-sectional study. J Clin Med 2020;9(6):1720. doi:10.3390/jcm9061720.
- 24.Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol 2019;10:1476. doi:10.3389/fphys.2019.01476.
- 25.Franzmann EJ, Donovan MJ. Effective early detection of oral cancer using a simple and inexpensive point of care device in oral rinses. Expert Rev Mol Diagn 2018;18(10):837-44. doi: 10.1080/14737159.2018.1523008.
- 26.Kaur J, Jacobs R, Huang Y, Salvo N, Politis C. Salivary biomarkers for oral cancer and pre-cancer screening: a review. Clin Oral Investig 2018;22(2):633-40. doi: 10.1007/s00784-018-2337-x.
- 27.Roi A, Rusu LC, Roi CI, Luca RE, Boia S, Munteanu RI. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers 2019:8761860.
- 28.Cheng YL, Jordan L, Chen HS, Kang D, Oxford L, Plemons J, et al. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J Periodontal Res 2017;52(3):428-37. doi: 10.1111/jre.12407.
- 29.Csősz É, Lábiscsák P, Kalló G, Márkus B, Emri M, Szabó A, et al. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS One 2017;12(5):e0177282. doi:10.1371/journal.pone.0177282.
- 30.Lee WH, Chen HM, Yang SF, Liang C, Peng CY, Lin FM, et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep 2017;7(1):16540. doi: 10.1038/s41598-017-16418-x.
- 31.Stuani VT, Rubira CM, Sant’Ana AC, Santos PS. Salivary biomarkers as tools for oral squamous cell carcinoma diagnosis: A systematic review. Head Neck 2017;39(4):797-811. doi: 10.1002/hed.24650.
- 32.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107(6):djv086.
- 33.Tang KD, Vasani S, Taheri T, Walsh LJ, Hughes BGM, Kenny L, Punyadeera C. An occult HPV-driven oropharyngeal squamous cell carcinoma discovered through a saliva test. Front Oncol 2020;10:408. doi: 10.3389/fonc.2020.00408.
- 34.OralDNA. OraRisk HPV. https://www.oraldna.com/test/ohpv-complete/
- 35.Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med 2015;7:293ra104. doi: 10.1126/scitranslmed.aaa8507.
- 36.Zielińska K, Karczmarek-Borowska B, Kwaśniak K, Czarnik-Kwaśniak J, Ludwin A, Lewandowski B, Tabarkiewicz J. Salivary IL-17A, IL-17F, and TNF-α are associated with disease advancement in patients with oral and oropharyngeal cancer. J Immunol Res 2020:3928504. doi: 10.1155/2020/3928504.
- 37.Lee LT, Wong YK, Hsiao HY, Wang YW, Chan MY, Chang KW. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2018;47(6):699-707. doi: 10.1016/j.ijom.2017.09.016.
- 38.Shen S, Saito Y, Ren S, Liu C, Guo T, Qualliotine J, et al. Targeting viral DNA and promoter hypermethylation in salivary rinses for recurrent HPV-positive oropharyngeal cancer. Otolaryngol Head Neck Surg 2020;162(4):512-9. doi: 10.1177/0194599820903031.
- 39.Hanna GJ, Lau CJ, Mahmood U, Supplee JG, Mogili AR, Haddad RI, et al. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol 2019;95:120-6. doi: 10.1016/j.oraloncology.
- 40.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am 2011;55(1):159-78. doi:10.1016/j.cden.2010.08.004.
- 41.Collins FM. Integrating the 4Ps into patient care. Available at: https://www.colgateoralhealthnetwork.com/article/integrating-the-4ps-into-patient-care/.
- 42.Knight ET, Murray Thomson W. A public health perspective on personalized periodontics. Periodontol 2000. 2018 Oct;78(1):195-200. doi: 10.1111/prd.12228. PMID: 30198135.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2020/11/salivary-diagnostics-4.jpg)