Smokeless Tobacco
Tobacco products have a dramatically adverse effect on human health, including oral health.1US Department of Health and Human Services. 50 years of progress: A report of the surgeon general, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/50-years-of-progress-bysection.html While generally considered less hazardous to health than smoked tobacco, smokeless tobacco (SLT) is associated with significant unfavorable effects on systemic and oral health. Recent developments, including the introduction of more novel forms of SLT and in some regions the widespread use of SLT containing betel quid and/or areca nut, are significant public health concerns.2Leon ME, Lugo A, Boffetta P, Gilmore A, Ross H, Schüz J, La Vecchia C, Gallus S. Smokeless tobacco use in Sweden and other 17 European countries. Eur J Pub Health 2016;26(5):817-21.,3Wiener RC. Association of smokeless tobacco use and smoking in adolescents in the United States. An analysis of data from the Youth Risk Behavior Surveillance System survey, 2011. J Am Dent Assoc 2013;144(8):930-8.,4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009.
Types of Smokeless Tobacco
Chew tobacco can be held in the vestibule of the mouth for several hours as a ‘wad,’ while dry or moist oral snuff is tucked under the upper or lower lip. Swedish snus is sold in small packets/sachets that can be placed directly under the lip. Oral SLT is also available as sticks or pellets, and is sold as a thin film that is placed on the tongue where it then dissolves.3Wiener RC. Association of smokeless tobacco use and smoking in adolescents in the United States. An analysis of data from the Youth Risk Behavior Surveillance System survey, 2011. J Am Dent Assoc 2013;144(8):930-8. (Table 1)
| Table 1. Main types of SLT | |
|---|---|
| Chew tobacco | Air- or fire-cured tobacco leaves, flavorings, sugars and other additives |
| Dry snuff (sniffed) | Air- or fire-cured fermented powdered tobacco |
| Moist snuff | Air- or fire-cured tobacco as fine particles |
| Swedish snus | Pasteurized unfermented tobacco, cherry and other flavors; no sugar |
| Paan | Betel quid, areca nut, quenched lime, seeds and spices |
| Gutkha | areca nut, slaked lime, spices and catechu |
In South Asia, SLT is a common and culturally significant practice, and is primarily sold as paan and gutkha. (Figure 1) Paan typically contains areca nut, betel quid, quenched lime, seeds and spices and gutkha typically contains areca nut, slaked lime, spices and catechu.4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009. SLT used in the Middle East and South Asia variously contains ash, sodium carbonate, indigo, spices, cotton, sesame oil, and molasses.4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009.

Figure 1.
Ingredients for Paan: betel leaves, nuts and lime
Source: Wikimedia Commons, Public Domain
Prevalence of Use
Globally, an estimated 300 million people use SLT, 90% of whom reside in South East Asia.6Freedman ND. Invited commentary: Smokeless tobacco-an important contributor to cancer, but more work is needed. Am J Epidemiol 2016;184(10):717-9. In the United States, an estimated 2.6% of American adults (4.8% of men and 0.3% of women) use SLT,7Agaku IT, King BA, Husten CG, Bunnell R, Ambrose BK, Hu SS, Holder-Hayes E, Day HR; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012–2013. Morb Mortal Wkly Rep 2014;63(25):542-7. and in one survey 9.9% and 1.2% of male and female high school students, respectively, reported using SLT in the prior 30 days.8Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS; Centers for Disease Control and Prevention (CDC). Tobacco use among middle and high school students—United States, 2011–2014. Morb Mortal Wkly Rep 2015;64(14):381-5. In addition, 12.3% of the Swedish population ages 16 and over (20.7% and 3.5% of men and women, respectively) is estimated to use SLT, mainly Swedish snus, compared to 1.1% of adults across 17 other countries in Europe.2Leon ME, Lugo A, Boffetta P, Gilmore A, Ross H, Schüz J, La Vecchia C, Gallus S. Smokeless tobacco use in Sweden and other 17 European countries. Eur J Pub Health 2016;26(5):817-21. However, in India, Nepal, Myanmar and Bangladesh, SLT use ranges from 20% to 80% of the adult population, and approximately three-quarters of Pakistani school students in one study reported using SLT.4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009.,9Eriksen M, Mackay J, Schluger N, Gomeshtapeh FI, Drope J. The tobacco atlas. 5th ed., 2015. The American Cancer Society. Available at: http://3pk43x313ggr4cy0lh3tctjh.wpengine.netdna-cdn.com/wp-content/uploads/2015/03/TA5_2015_WEB.pdf. In African and Middle Eastern countries, use of SLT among adults has been reported to range from less than 1% to 20%.9Eriksen M, Mackay J, Schluger N, Gomeshtapeh FI, Drope J. The tobacco atlas. 5th ed., 2015. The American Cancer Society. Available at: http://3pk43x313ggr4cy0lh3tctjh.wpengine.netdna-cdn.com/wp-content/uploads/2015/03/TA5_2015_WEB.pdf. (Table 2)
| Table 2. Prevalence of use of SLT among adults | |
|---|---|
| United States | 2.6% |
| Sweden | 12.3% |
| Africa and The Middle East | <1% to 20% |
| India, Nepal, Myanmar and Bangladesh | 20% to 80% |
Noxious Substances in SLT
In a comparison of American ‘snus’ (moist snuff) and Swedish snus, the latter had considerably higher proportions of un-ionized nicotine
Impact on Systemic Health
Recently, a study was published examining the association of SLT with all- and specific-cause mortality.15Timberlake DS, Nikitin D, Johnson NJ, Altekruse SF. A longitudinal study of smokeless tobacco use and mortality in the United States. Int J Cancer 2017;141:264-70. Based on data from the National Longitudinal Mortality Study conducted in the United States from 1985 to 2011, the study found an increased risk of death due to coronary artery disease for male moist snuff users compared to never-users of SLT and never-smokers. No increased risk was observed for all cancers, digestive cancers or cerebrovascular disease. However, SLT is considered causal for esophageal and pancreatic cancer.6Freedman ND. Invited commentary: Smokeless tobacco-an important contributor to cancer, but more work is needed. Am J Epidemiol 2016;184(10):717-9. A second study noted a two-fold risk of hypercholesterolemia for smokers and heavy SLT users compared to nonusers of tobacco products, and in a separate review SLT use was reported as a risk factor for cardiovascular disease, as well as increased mortality.16Bolinder G. Overview of knowledge of health effects of smokeless tobacco. Increased risk of cardiovascular diseases and mortality because of snuff. Lakartidningen 1997;94(42):3725-31.,17Tucker LA. Use of smokeless tobacco, cigarette smoking, and hypercholesterolemia. Am J Public Health 1989;79(8):1048-50.
Impact on Oral Health
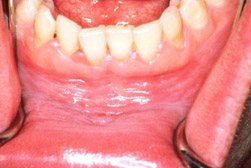
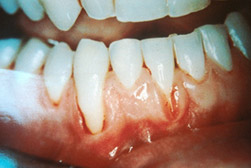
SLT has a significant impact on oral health, especially with respect to the risk for pre-malignant lesions and malignancies. The relationship between SLT and oral pre-malignant and malignant lesions was recently investigated in a pooled analysis of eleven US studies conducted between 1981 and 2006, with more than 6,000 cases and 8,000 controls.18Wyss AB, Hashibe M, Lee YA, Chuang S-C, Muscat J, Chen C, Schwartz SM, Smith E, Zhang Z-F, Morgenstern H, Wei Q, Li G, Kelsey KT, McClean M, Winn DM, Schantz S, Yu G-P, Gillison ML, Zevallos JP, Boffetta P, Olshan AF. Smokeless tobacco use and the risk of head and neck cancer: Pooled analysis of US studies in the INHANCE consortium. Am J Epidemiol 2016;184(10):703-16. A three-fold and approximately two-fold risk of oral cancers was found for users of snuff and chew tobacco, respectively, compared to nonusers of SLT or cigarettes. Further, a high prevalence of leukoplakia has been observed at the sites where SLT is held in the oral cavity.19Robertson PB, Walsh MM, Greene JC. Oral effects of smokeless tobacco use by professional baseball players. Adv Dent Res 1997;11(3):307-12. SLT containing sugar increases risk for dental caries, specifically, at the intraoral site where it is retained.20Going RE, Hsu SC, Pollack RL, Haugh LD. Sugar and fluoride content of various forms of tobacco. J Am Dent Assoc 1980;100:27-33.,21Tomar SL, Winn DM. Chewing tobacco use and dental caries among US men. J Am Dent Assoc 1999;130(11):1601-10. In addition, SLT is a risk factor for gingivitis, halitosis, staining and localized attachment loss.5Savitz DA, Meyer RE, Tanzer JM, Mirvish SS, Lewin F. Public health implications of smokeless tobacco use as a harm reduction strategy. Am J Public Health 2006;96(11):1934-9.,22Kallischnigg G, Weitkunat R, Lee PN. Systematic review of the relation between smokeless tobacco and non-neoplastic oral diseases in Europe and the United States. BMC Oral Health 2008;8:13. (Figures 2, 3)
The development of potentially malignant oral lesions associated with the use of SLT in South Asia is a particularly ominous public health problem.4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009.,23Seenan P, Conway D. Smokeless tobacco – a substantial risk for oral potentially malignant disorders in South Asia. Evid Based Dent 2017;18:54-5. Of further concern, as smoking has declined the use of SLT in this region has increased.24Suliankatchi RA, Sinha DN, Rath R, Aryal KK, Zaman MM, Gupta PC, Karki KB, Venugopal D. Smokeless tobacco use is ‘replacing’ the smoking epidemic in the South East Asia Region. Nicotine Tob Res 2017 Dec 22. doi: 15.1093/ntr/ntx272. [Epub ahead of print] Betel quid and areca nut enhance the adverse effects of SLT, and are themselves associated with pre-malignant and malignant change.5Savitz DA, Meyer RE, Tanzer JM, Mirvish SS, Lewin F. Public health implications of smokeless tobacco use as a harm reduction strategy. Am J Public Health 2006;96(11):1934-9. Other ingredients also enhance risk for oral cancer, including catechu which is strongly associated with esophageal cancer.4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009. Paan and gutkha also result in oral submucous fibrosis (OSMF), a chronic and progressive inflammatory precancerous condition involving fibrosis of connective tissues.4Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 2017;39:e2017009. The reported incidence for OSMF is 75% after 4.5 years of paan use. In addition, lime contained in SLT can trigger tissue irritation and hyperplasia.
Implications for Oral Health Professionals
Conclusions
Dental professionals must be strong advocates for avoidance of all tobacco products, including SLT. Given the known oral and systemic health risks associated with SLT, policies should be introduced and strengthened to reduce SLT usage and to prevent it replacing smoked tobacco.24Suliankatchi RA, Sinha DN, Rath R, Aryal KK, Zaman MM, Gupta PC, Karki KB, Venugopal D. Smokeless tobacco use is ‘replacing’ the smoking epidemic in the South East Asia Region. Nicotine Tob Res 2017 Dec 22. doi: 15.1093/ntr/ntx272. [Epub ahead of print] In addition, more research is required to fully assess the adverse health effects associated with use of SLT,6Freedman ND. Invited commentary: Smokeless tobacco-an important contributor to cancer, but more work is needed. Am J Epidemiol 2016;184(10):717-9. alone as well as in combination with other tobacco products.6Freedman ND. Invited commentary: Smokeless tobacco-an important contributor to cancer, but more work is needed. Am J Epidemiol 2016;184(10):717-9.,18Wyss AB, Hashibe M, Lee YA, Chuang S-C, Muscat J, Chen C, Schwartz SM, Smith E, Zhang Z-F, Morgenstern H, Wei Q, Li G, Kelsey KT, McClean M, Winn DM, Schantz S, Yu G-P, Gillison ML, Zevallos JP, Boffetta P, Olshan AF. Smokeless tobacco use and the risk of head and neck cancer: Pooled analysis of US studies in the INHANCE consortium. Am J Epidemiol 2016;184(10):703-16.
References
- 1.Dominy SS, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333 https://www.ncbi.nlm.nih.gov/pubmed/30746447.
- 2.Sadrameli M, et al. Linking mechanisms of periodontitis to Alzheimer’s disease. Curr Opin Neurol. 2020;33(2):230-8 https://www.ncbi.nlm.nih.gov/pubmed/32097126.
- 3.Borsa L, et al. Analysis the link between periodontal diseases and Alzheimer’s disease: A systematic review. Int J Environ Res Public Health. 2021;18(17) https://www.ncbi.nlm.nih.gov/pubmed/34501899.
- 4.Costa MJF, et al. Relationship of Porphyromonas gingivalis and Alzheimer’s disease: A systematic review of pre-clinical studies. Clin Oral Investig. 2021;25(3):797-806 https://www.ncbi.nlm.nih.gov/pubmed/33469718.
- 5.Munoz Fernandez SS, Lima Ribeiro SM. Nutrition and Alzheimer disease. Clin Geriatr Med. 2018;34(4):677-97 https://www.ncbi.nlm.nih.gov/pubmed/30336995.
- 6.Aquilani R, et al. Is the Brain Undernourished in Alzheimer’s Disease? Nutrients. 2022;14(9) https://www.ncbi.nlm.nih.gov/pubmed/35565839.
- 7.Fukushima-Nakayama Y, et al. Reduced mastication impairs memory function. J Dent Res. 2017;96(9):1058-66 https://www.ncbi.nlm.nih.gov/pubmed/28621563.
- 8.Kim HB, et al. Abeta accumulation in vmo contributes to masticatory dysfunction in 5XFAD Mice. J Dent Res. 2021;100(9):960-7 https://www.ncbi.nlm.nih.gov/pubmed/33719684.
- 9.Miura H, et al. Relationship between cognitive function and mastication in elderly females. J Oral Rehabil. 2003;30(8):808-11 https://www.ncbi.nlm.nih.gov/pubmed/12880404.
- 10.Lexomboon D, et al. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60(10):1951-6 https://www.ncbi.nlm.nih.gov/pubmed/23035667.
- 11.Elsig F, et al. Tooth loss, chewing efficiency and cognitive impairment in geriatric patients. Gerodontology. 2015;32(2):149-56 https://www.ncbi.nlm.nih.gov/pubmed/24128078.
- 12.Kim EK, et al. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch Gerontol Geriatr. 2017;70:209-13 https://www.ncbi.nlm.nih.gov/pubmed/28214402.
- 13.Kim MS, et al. The association between mastication and mild cognitive impairment in Korean adults. Medicine (Baltimore). 2020;99(23):e20653 https://www.ncbi.nlm.nih.gov/pubmed/32502052.
- 14.Cardoso MG, et al. Relationship between functional masticatory units and cognitive impairment in elderly persons. J Oral Rehabil. 2019;46(5):417-23 https://www.ncbi.nlm.nih.gov/pubmed/30614023.
- 15.Popovac A, et al. Oral health status and nutritional habits as predictors for developing alzheimer’s disease. Med Princ Pract. 2021;30(5):448-54 https://www.ncbi.nlm.nih.gov/pubmed/34348313.
- 16.Park T, et al. More teeth and posterior balanced occlusion are a key determinant for cognitive function in the elderly. Int J Environ Res Public Health. 2021;18(4) https://www.ncbi.nlm.nih.gov/pubmed/33669490.
- 17.Lin CS, et al. Association between tooth loss and gray matter volume in cognitive impairment. Brain Imaging Behav. 2020;14(2):396-407 https://www.ncbi.nlm.nih.gov/pubmed/32170642.
- 18.Kumar S, et al. Oral health status and treatment need in geriatric patients with different degrees of cognitive impairment and dementia: a cross-sectional study. J Family Med Prim Care. 2021;10(6):2171-6 https://www.ncbi.nlm.nih.gov/pubmed/34322409.
- 19.Delwel S, et al. Chewing efficiency, global cognitive functioning, and dentition: A cross-sectional observational study in older people with mild cognitive impairment or mild to moderate dementia. Front Aging Neurosci. 2020;12:225 https://www.ncbi.nlm.nih.gov/pubmed/33033478.
- 20.Da Silva JD, et al. Association between cognitive health and masticatory conditions: a descriptive study of the national database of the universal healthcare system in Japan. Aging (Albany NY). 2021;13(6):7943-52 https://www.ncbi.nlm.nih.gov/pubmed/33739304.
- 21.Galindo-Moreno P, et al. The impact of tooth loss on cognitive function. Clin Oral Investig. 2022;26(4):3493-500 https://www.ncbi.nlm.nih.gov/pubmed/34881401.
- 22.Stewart R, et al. Adverse oral health and cognitive decline: The health, aging and body composition study. J Am Geriatr Soc. 2013;61(2):177-84 https://www.ncbi.nlm.nih.gov/pubmed/23405916.
- 23.Dintica CS, et al. The relation of poor mastication with cognition and dementia risk: A population-based longitudinal study. Aging (Albany NY). 2020;12(9):8536-48 https://www.ncbi.nlm.nih.gov/pubmed/32353829.
- 24.Kim MS, Han DH. Does reduced chewing ability efficiency influence cognitive function? Results of a 10-year national cohort study. Medicine (Baltimore). 2022;101(25):e29270 https://www.ncbi.nlm.nih.gov/pubmed/35758356.
- 25.Ko KA, et al. The Impact of Masticatory Function on Cognitive Impairment in Older Patients: A Population-Based Matched Case-Control Study. Yonsei Med J. 2022;63(8):783-9 https://www.ncbi.nlm.nih.gov/pubmed/35914761.
- 26.Garre-Olmo J. [Epidemiology of Alzheimer’s disease and other dementias]. Rev Neurol. 2018;66(11):377-86 https://www.ncbi.nlm.nih.gov/pubmed/29790571.
- 27.Stephan BCM, et al. Secular Trends in Dementia Prevalence and Incidence Worldwide: A Systematic Review. J Alzheimers Dis. 2018;66(2):653-80 https://www.ncbi.nlm.nih.gov/pubmed/30347617.
- 28.Lopez OL, Kuller LH. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer’s disease and other dementias. Handb Clin Neurol. 2019;167:139-48 https://www.ncbi.nlm.nih.gov/pubmed/31753130.
- 29.Ono Y, et al. Occlusion and brain function: mastication as a prevention of cognitive dysfunction. J Oral Rehabil. 2010;37(8):624-40 https://www.ncbi.nlm.nih.gov/pubmed/20236235.
- 30.Kubo KY, et al. Masticatory function and cognitive function. Okajimas Folia Anat Jpn. 2010;87(3):135-40 https://www.ncbi.nlm.nih.gov/pubmed/21174943.
- 31.Chen H, et al. Chewing Maintains Hippocampus-Dependent Cognitive Function. Int J Med Sci. 2015;12(6):502-9 https://www.ncbi.nlm.nih.gov/pubmed/26078711.
- 32.Azuma K, et al. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int J Mol Sci. 2017;18(8) https://www.ncbi.nlm.nih.gov/pubmed/28771175.
- 33.Chuhuaicura P, et al. Mastication as a protective factor of the cognitive decline in adults: A qualitative systematic review. Int Dent J. 2019;69(5):334-40 https://www.ncbi.nlm.nih.gov/pubmed/31140598.
- 34.Lopez-Chaichio L, et al. Oral health and healthy chewing for healthy cognitive ageing: A comprehensive narrative review. Gerodontology. 2021;38(2):126-35 https://www.ncbi.nlm.nih.gov/pubmed/33179281.
- 35.Tada A, Miura H. Association between mastication and cognitive status: A systematic review. Arch Gerontol Geriatr. 2017;70:44-53 https://www.ncbi.nlm.nih.gov/pubmed/28042986.
- 36.Ahmed SE, et al. Influence of Dental Prostheses on Cognitive Functioning in Elderly Population: A Systematic Review. J Pharm Bioallied Sci. 2021;13(Suppl 1):S788-S94 https://www.ncbi.nlm.nih.gov/pubmed/34447202.
- 37.Tonsekar PP, et al. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology. 2017;34(2):151-63 https://www.ncbi.nlm.nih.gov/pubmed/28168759.
- 38.Nangle MR, Manchery N. Can chronic oral inflammation and masticatory dysfunction contribute to cognitive impairment? Curr Opin Psychiatry. 2020;33(2):156-62 https://www.ncbi.nlm.nih.gov/pubmed/31895157.
- 39.Nakamura T, et al. Oral dysfunctions and cognitive impairment/dementia. J Neurosci Res. 2021;99(2):518-28 https://www.ncbi.nlm.nih.gov/pubmed/33164225.
- 40.Weijenberg RAF, et al. Mind your teeth-The relationship between mastication and cognition. Gerodontology. 2019;36(1):2-7 https://www.ncbi.nlm.nih.gov/pubmed/30480331.
- 41.Asher S, et al. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J Am Geriatr Soc. 2022;70(9):2695-709 https://www.ncbi.nlm.nih.gov/pubmed/36073186.
- 42.Lin CS. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr. 2018;18(1):5 https://www.ncbi.nlm.nih.gov/pubmed/29304748.
- 43.Wu YT, et al. The changing prevalence and incidence of dementia over time – current evidence. Nat Rev Neurol. 2017;13(6):327-39 https://www.ncbi.nlm.nih.gov/pubmed/28497805.
- 44.National Psoriasis Foundation. Soriatane (Acitretin). https://www.psoriasis.org/soriatane-acitretin/.
- 45.National Psoriasis Foundation. Current Biologics on the Market. https://www.psoriasis.org/current-biologics-on-the-market/.
- 46.Dalmády S, Kemény L, Antal M, Gyulai R. Periodontitis: a newly identified comorbidity in psoriasis and psoriatic arthritis. Expert Rev Clin Immunol 2020;16(1):101-8. doi: 10.1080/1744666X.2019.1700113.
- 47.Mickenautsch S, Yengopal V. Effect of xylitol versus sorbitol: a quantitative systematic review of clinical trials. Int Dent J 2012;62(4):175-88.
- 48.Rethman MP, Beltrán-Aguilar ED, Billings RJ, Burne RA, Clark M, Donly KJ, Hujoel PP, Katz BP, Milgrom P, Sohn W, Stamm JW, Watson G, Wolff M, Wright T, Zero D, Aravamudhan K, Frantsve-Hawley J, Meyer DM; for the American Dental Association Council on Scientific Affairs Expert Panel on Nonfluoride Caries-Preventive Agents. Nonfluoride caries-preventive agents. Executive summary of evidence-based clinical recommendations. J Am Dent Assoc 2011;142(9):1065-71.
- 49.Milgrom P, Söderling EM, Nelson S, Chi DL, Nakai Y. Clinical evidence for polyol efficacy. Adv Dent Res 2012; 24(2):112-6.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2023/05/Ira-Lamster-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2018/07/smokeless-tabacco-2.jpg)