The Oral Cavity and Inflammatory Bowel Disease
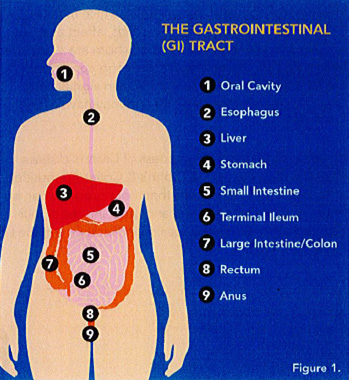
The gastrointestinal (GI) system begins at the mouth and continues inferiorly to include the esophagus, stomach, small intestine and large intestine, rectum and terminates at the anus (Figure 1). This organ system takes in food and drink, and processes what is ingested, extracting nutrients, thereby providing the energy source for the body. Other organs such as the liver are often considered when discussing the GI system.
The initial digestion of food occurs in the mouth via both the mechanical breakdown (mastication) and the action of enzymes in saliva such as amylase, which hydrolyzes starch into sugar.
The GI system functions to maintain homeostasis, promoting health and well-being. However, certain disease processes are recognized to affect different parts of the GI system, and abnormalities of one part of the system can affect other parts.
A recent investigation examined molecular mechanisms that can link oral diseases, specifically periodontitis, and colitis, which is inflammation of the large intestine near the terminus of the GI track1Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG, 3rd, Hayashi A, Imai J, et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182(2):447-62 e14.. This comprehensive study utilized different mouse models to determine if the inflammatory response in the periodontal tissues can contribute to the development of inflammatory bowel disease (IBD). Two mechanisms were identified. First, as periodontitis develops, the periodontal microflora becomes more complex, some of the pathogens are swallowed, travelling down to the colon and activating monocytes, thereby triggering a more intense inflammatory response. In addition, certain lymphocytes in the periodontal tissues, known as T helper lymphocytes, respond specifically to the pathogenic bacteria, which are also referred to as pathobionts. These T helper lymphocytes then home to the gut and upon encountering the specific oral bacteria will activate and contribute to gut inflammation. These specific lymphocytes are not activated by the normal gut bacteria.
The inflammatory molecule that was identified as responsible for the development of gut inflammation is interleukin-1β, which has been shown to play an important role as in the pathogenesis of periodontitis2Murakami T, Takahata Y, Hata K, Nishimura R. Role of interleukin-1 and inflammasomes in oral disease. J Oral Biosci. 2020;62(3):242-8., and has also been associated with the linkage of periodontitis and certain chronic disease3Geismar K, Enevold C, Sorensen LK, Gyntelberg F, Bendtzen K, Sigurd B, et al. Involvement of interleukin-1 genotypes in the association of coronary heart disease with periodontitis. J Periodontol. 2008;79(12):2322-30..
The new experimental evidence demonstrates specific mechanisms that link periodontitis to the development IBD leads to a broader discussion of how IBD can be influenced by oral diseases, and how oral disease can influence IBD. This topic is not often considered when the relationship of oral infection/oral inflammation (periodontitis, endodontic infections) to systemic diseases/chronic diseases is discussed.
Since IBD is not uncommon, these patients will be encountered in clinical practice. All oral healthcare providers need to be aware of these associations.
Inflammatory Bowel Disease
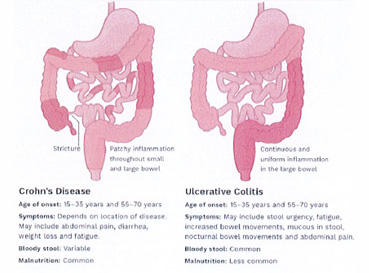
IBD is a general term used to describe chronic inflammatory disorders of the GI track. IBD is divided into two entities: ulcerative colitis (UC) and Crohn’s disease (CD). UC is a chronic condition of the colon and rectum, characterized by ulcer formation. CD is an inflammatory disorder that can involve any aspect of the GI track, and the ulcers that form can extend deeper into the lining of the GI track (Figure 2). UC and CD have no sex predilection, most often occur between the ages of 14 and 23, and in the United States UC affects more than 900,000 individuals, while CD affects more than 780,000 people. In both disorders, other areas of the body can be affected, including the eyes, joints and skin4Https://www.Crohnsandcolitis.Com. Accessed october 8, 2020..(Table 1)
A review of the oral manifestations of IBD identified 17 reports that include patients with CD and UC5Lauritano D, Boccalari E, Di Stasio D, Della Vella F, Carinci F, Lucchese A, et al. Prevalence of oral lesions and correlation with intestinal symptoms of inflammatory bowel disease: A systematic review. Diagnostics (Basel). 2019;9(3).. A wide range in prevalence of oral manifestation of IBD was reported for both adults (0.7% to 37%) and children (7.3% to 23%). The highest prevalence of oral manifestations was reported for children with CD (41%). The authors attributed the variation in prevalence to heterogeneous study design, including the population studied, who was identifying the oral lesions (physicians/gastroenterologists or dentists) and the criteria for identifying lesions. Nevertheless, a lengthy list of both specific and nonspecific oral lesions associated with UC and CD was developed.
Table 1: Oral lesions associated with IBD
| Non-specific lesions | Oral finding | UC | CD |
|---|---|---|---|
| Specific lesions | Cobblestone mucosa | √ | |
| Granulomatous cheilitis | √ | ||
| Mucosal tissue tags | √ | ||
| Pyostomatitis Vegetans | √ | ||
| Non-specific lesions | Deep oral fissures | √ | |
| Angular cheilitis | √ | √ | |
| Dental caries | √ | √ | |
| Mucogingivitis | √ | √ | |
| Periodontitis | √ | √ | |
| Lichen planus | √ | ||
| Dysphagia (difficult swallowing) | √ | √ | |
| Dry mouth | √ | √ | |
| Halitosis | √ | √ | |
| Altered taste | √ | √ | |
| Aphthous Ulcers | √ | √ |
(Modified from Lauritano et al. 2019)
Many of these findings were asymptomatic, but it was noted that between 12.7% and 21% of the lesions were identified before the diagnosis of IBD was made. A particular important problem is the occurrence of aphthous ulcers, which are similar to the ulcers occurring in the lower GI tract. These ulcers are painful, generally have a red border and recur over time. These ulcers are particularly problematic for children, as they can interfere with food intake. An association of these aphthous ulcers and active phases of the IBD has been suggested but is not fully defined. Nevertheless, the appearance of these lesions may necessitate a referral to the treating gastroenterologist.
Another lesion that has received attention in the literature is the cobblestone appearance of the mucosa occasionally seen in patients with CD. Linear, indented ulcerations separated by normal mucosa has the appearance of cobblestones. This is not an uncommon finding in persons with CD, with a reported prevalence of 6% in children and 20% in adults5Lauritano D, Boccalari E, Di Stasio D, Della Vella F, Carinci F, Lucchese A, et al. Prevalence of oral lesions and correlation with intestinal symptoms of inflammatory bowel disease: A systematic review. Diagnostics (Basel). 2019;9(3).. Persons with CD can also demonstrate swelling of the oral mucosa, most often on the buccal surface and in the retromolar area, as well as extra-orally, affecting the face and lips. If the lips are involved, painful fissures can develop. If occurring extra-orally, these swellings can present an esthetic concern for patients. Histologic examination of these swellings suggests a granulomatous inflammation6Mejia LM. Oral manifestations of gastrointestinal disorders. Atlas Oral Maxillofac Surg Clin North Am. 2017;25(2):93-104..
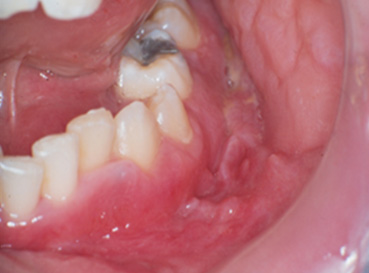
Pyostomatitis vegetans is a rare but potentially important oral finding in patients with UC but has also been detected in patients with CD (Figure 3). This lesion has been identified by a “snail track” appearance of the mucosa, as a surface layer of small pustules become eroded, resulting in ulcer formation. These lesions have been reported on all oral mucosal surfaces (vestibules, tongue, gingiva and palate;7Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22(25):5655-67.).
When evaluating patients with IBD, clinicians should be aware of secondary oral manifestations associated with these diseases. Specifically, patients may present with nutritional deficiencies, Further, medications used in treatment of IBD may have oral side effects 7Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22(25):5655-67..
The Effect of Malnutrition7Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22(25):5655-67.
It had been estimated that nearly a quarter of IBD outpatients are malnourished, while more than 4 of 5 hospitalized IBD patients suffer from this condition. The reasons: food intake is limited, absorption of nutrients is compromised, as a side effect of drugs, and due to previous surgery to remove parts of the GI track.
The most common type of problem is anemia as a result of reduced intake of iron, vitamin B12 and/or folate. Iron deficiency anemia can manifest in the oral cavity as pale and atrophic mucosa. The tongue can also appear atrophic and be painful. Anemia associated with a deficiency of vitamin B12 has been associated with atrophy of both the oral mucosa and tongue (which also can be painful), oral ulcerations, different clinical presentations of Candida infection, a generalized erythema of the oral mucosa, and the palatal mucosa can have a yellowish tinge. Neurological-related symptoms include altered taste and a burning sensation in the mouth. Anemia related to a folate deficiency can be associated with oral findings that are comparable to what is seen in vitamin B12 deficiency, but without neurological manifestations.
Other vitamin deficiencies can occur in IBD patients. If vitamin D is deficient than over time the affected person may experience skeletal/osseous abnormalities related to hypomineralization, and therefore a risk of bone fracture. Other factors may contribute to the increased risk of fracture, including malabsorption and/or deficiency of calcium. In children, such deficiencies can also lead to hypomineralization of the teeth. Vitamin A and C deficiencies have also been reported in IBD patients. Oral manifestations of these deficiencies can lead to mucosal atrophy, and mouth dryness. Increased risk of periodontal disease and gingival inflammation with bleeding and tissue swelling may also occur. In adults, such disorders are seen with severe, long-standing deficiencies of vitamin C. Lastly, zinc deficiency has been commonly reported in patients with CD. Oral manifestations can include ulcerations and erosion, a crusty appearance of the lips as well as both burning mouth and taste alteration.
The Effect of Medications Used to Treat IBD7Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22(25):5655-67.
There are several types/classes of drugs used to treat patients with IBD, and these can be associated with oral side effects.
Aminosalicylates: Salicylates are drugs that have an anti-inflammatory effect, and sulfasalazine has been used in the treatment of UC for more than half a century. Side effects are not common but can include development of a lichenoid-like lesion (lichen planus) of the mucosa. Patients may also complain of a metallic taste in their mouth.
Corticosteroids: are utilized in IBD for their anti-inflammatory effects. These drugs are not used for the long-term due to their associated side effects, which includes immunosuppressive. Well-recognized side effects of extended use of corticosteroids that occur in the head and neck region include a moon-face appearance and acne. A common oral complication of these drugs is overgrowth of Candida, which can occur with different presentations (i.e. pseudomembranous, atrophic). Viral infections can also occur (i.e. Herpes zoster, Herpes simplex). Use of these drugs is generally contraindicated in children due to adverse effects on bone growth.
Thiopurines: have an anti-inflammatory effect, and azathioprine and 6-mercaptopurine are used in the treatment of IBD, often as maintenance therapy. The precise mechanism of action has not been defined, but these drugs have also been used for decades. Side effects that can manifest in the oral cavity can include various taste disturbances, opportunistic infections, including Herpes simplex virus infection. Long term use has been associated with development of certain cancers including lymphoma.
Methotrexate: is a chemotherapeutic agent and is often used to treat malignancy. It has been used in IBD for its immunosuppressive effects. Methotrexate is toxic for the mucosa and can be associated with oral ulcerations (at a lower dose) and mucositis (at a higher dose). At higher doses methotrexate can suppress bone marrow, which can lead to oral manifestations related to a decrease in leukocytes and erythrocytes.
Cyclosporin: has immunosuppression effects and is associated with gingival overgrowth. Overgrowth is influenced by how long the drug is used as well as local factors (i.e. plaque accumulation). Other possible oral side-effects include Candida infection and hyperplasia of filiform papillae of the tongue.
As new and other agents are introduced for the treatment of IBD, oral side effects will be identified. These new agents include monoclonal antibodies directed against specific inflammatory mediators such as tumor necrosis factor α. Oral side effects of these biological agents can include lichenoid reactions of the mucosa and opportunistic infections including Candida overgrowth.
Conclusions
For all too long, the oral cavity, and diseases and disorders of the teeth, supporting tissues of the dentition, and mucosal tissues have been considered as separate from the rest of the body. This situation is illogical for many reasons, but none more so than when considering the function of the gastrointestinal system.
This situation is now changing, and there is a growing appreciation of the importance of the oral cavity as a functional unit in the maintenance of homeostasis, how diseases of the oral cavity can influence systemic diseases, as well changes in the oral cavity that occur as a result of a number of systemic disorders.
It is incumbent upon every oral health care provider to have an appreciation for these relationships, as well as a general understanding of the physiology and pathology of different organ systems. A thorough medical history is essential, including a review of prescription and over-the-counter drug usage. This essay serves as an introduction/brief review of the oral cavity in the context of IBD.
References
- 1.Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG, 3rd, Hayashi A, Imai J, et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182(2):447-62 e14.
- 2.Murakami T, Takahata Y, Hata K, Nishimura R. Role of interleukin-1 and inflammasomes in oral disease. J Oral Biosci. 2020;62(3):242-8.
- 3.Geismar K, Enevold C, Sorensen LK, Gyntelberg F, Bendtzen K, Sigurd B, et al. Involvement of interleukin-1 genotypes in the association of coronary heart disease with periodontitis. J Periodontol. 2008;79(12):2322-30.
- 4.Https://www.Crohnsandcolitis.Com. Accessed october 8, 2020.
- 5.Lauritano D, Boccalari E, Di Stasio D, Della Vella F, Carinci F, Lucchese A, et al. Prevalence of oral lesions and correlation with intestinal symptoms of inflammatory bowel disease: A systematic review. Diagnostics (Basel). 2019;9(3).
- 6.Mejia LM. Oral manifestations of gastrointestinal disorders. Atlas Oral Maxillofac Surg Clin North Am. 2017;25(2):93-104.
- 7.Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22(25):5655-67.