Artificial Sweeteners
Humans are born with a liking for sweetness, which is believed to encourage consumption of maternal milk and fruits.1Ventura AK , Mennella JA. Innate and learned preferences for sweet taste during childhood. Curr Opin Clin Nutr Metab Care 2011;14(4):379-84.,2Murray RD. Savoring sweet: Sugars in infant and toddler feeding. Ann Nutr Metab 2017;70 (Suppl 3):38-46. Sugars are present naturally in some foods, while added sugars are used to impart sweetness and taste, adjust viscosity, volume, texture and appearance, and for preservation and fermentation.3Davis E. Functionality of sugars: Physicochemical interactions in foods. Am J Clin Nutr 1995;62(suppl):170S-7S.,4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,5Al Humaid J. Sweetener content and cariogenic potential of pediatric oral medications: A literature. Int J Health Sci (Qassim) 2018;12(3):75-82. However, high intake of sugars is associated with obesity, diabetes mellitus (DM), metabolic syndrome, cardiovascular disease (CVD) and dental caries.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58. Obesity and DM are already at epidemic levels globally, with an estimated 592 million patients with DM projected by 2035.6International Diabetes Federation. 6th edn. IDF; Brussels: 2013. Diabetes atlas. Available at:www.idf.org/diabetesatlas. Sugar substitutes are used as a cost-efficient method of obtaining sweetness, to help reduce caloric intake, improve glycemic control, and control dental caries.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,7Wee M, Tan V, Forde C. A comparison of psychophysical dose-response behaviour across 16 sweeteners. Nutrients 2018;10(11):pii E1632. They include non-nutritive and nutritive alternatives, both synthetic and natural. Consistent with common usage, non-nutritive sweeteners are referred to here as artificial sweeteners (AS).
AS and Regulatory Approval
Approval/acceptance of AS varies by country. In the United States, AS are regulated by the Food and Drug Administration (FDA) as approved food additives or generally recognized as safe (GRAS). They include acesulfame-K, advantame, aspartame, swingle/monk fruit extract (luo han guo), neotame, saccharin, refined stevia, and sucralose. (Table 1) Artificially sweetened beverages (ASBs) are the primary dietary source of AS in the US and globally.8Malek AM, Hunt KJ, DellaValle DM, Greenberg D, St. Peter JV, Marriott BP. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007–2012. Curr Dev Nutr 2018;2:nzy054.,9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40.
| Table 1. Artificial sweeteners in the United States4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,10Calorie Control Council. Statements on sweetener safety. Available at: www.caloriecontrolcouncil.org.,13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701. | ||||
|---|---|---|---|---|
| FDA status | Compared to sugar | Uses | Examples | |
| Acesulfame-K | Food additive | 180 to 200x sweeter | Foods, drinks, table sweetener, cosmetics, pharmaceuticals | Sunett; Sweet One |
| Advantame | GRAS | 20,000x sweeter | Foods, drinks | Advantame |
| Aspartame | Food additive | 160 to 200x sweeter | Foods, drinks, table sweetener, pharmaceuticals | Equal; NutraSweet; |
| Neotame | Food additive | 7,000 to 13,000x sweeter | Foods, drinks | NutraSweet Neotame |
| Refined Stevia | GRAS | 300 to 400x sweeter | Foods, drinks, table sweetener | Pure Via; Truvia; Enliten |
| Saccharin | Food additive | 200 to 700x sweeter | Foods, drinks, table sweetener, gums, dentifrices, cosmetics, pharmaceuticals | Sweet’n Low; Sweet Twin; Necta Sweet |
| Swingle fruit extract | GRAS | 150 to 250x sweeter | Foods, drinks, table sweetener | PureLo; Monk Fruit in the Raw |
| Sucralose | Food additive | 400x sweeter | Foods, drinks, table sweetener, chewing gum | Splenda |
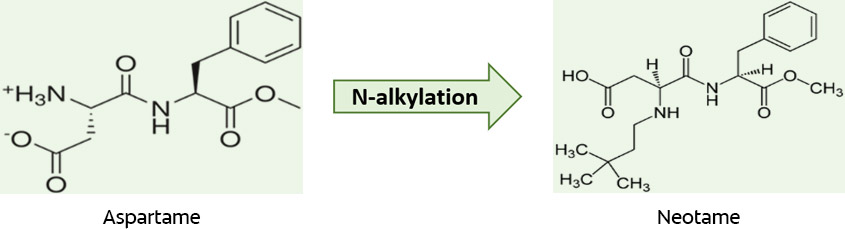
Acesulfame-K combines an organic acid and potassium and does not affect potassium levels.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58. Aspartame offers a clean taste, and flavor extension.10Calorie Control Council. Statements on sweetener safety. Available at: www.caloriecontrolcouncil.org. With more than 200 safety studies, it has been declared safe in >100 countries.11Lindseth GN, Coolahan SE, Petros TV, Lindseth PD. Neurobehavioral effects of aspartame consumption. Res Nurs Health 2014;37(3):185-93. After ingestion, it breaks down into phenylalanine, aspartic acid and methanol.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58. People born with phenylketonuria cannot metabolize phenylalanine, requiring restricted intake in youth and during pregnancy. Aspartame- and phenylalanine-containing foods and drinks must carry a warning that they contain phenylalanine. Caution is advised for individuals with tardive dyskinesia, sleep or anxiety disorders, other mental health conditions, or taking certain medications.12Mayo Clinic. Healthy Lifestyle. Nutrition and healthy eating. Available at: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/expert-answers/phenylalanine/faq-20058361. Advantame is derived from aspartame and vanillin, and Neotame is formed through N-alkylation of aspartame.10Calorie Control Council. Statements on sweetener safety. Available at: www.caloriecontrolcouncil.org. (Figure 1)
Saccharin is derived from an organic sulfa-based molecule.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701. Rats fed extreme doses of saccharin daily developed bladder cancer in studies, which resulted in saccharin being banned in 1981.14Touyz LZG. Saccharin deemed “not hazardous” in United States and abroad. Curr Oncol 2011;18(5):213-4. However, rats do not metabolize saccharin in the same manner as humans. Additionally, to reach an equivalent dose a human adult would have to consume 800 twelve-ounce diet sodas containing saccharin daily.14Touyz LZG. Saccharin deemed “not hazardous” in United States and abroad. Curr Oncol 2011;18(5):213-4. More than 30 human studies have since shown saccharin to be safe for consumption and >100 countries have approved saccharin, including the US.15United States Food & Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. Available at: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states#SummaryTable. Cross-sensitivity reactions may occur in children with sulfonamide allergy.5Al Humaid J. Sweetener content and cariogenic potential of pediatric oral medications: A literature. Int J Health Sci (Qassim) 2018;12(3):75-82. Refined stevia is derived from the herb ‘Stevia Rebaudiana Bertoni.’4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701. Reported side effects include mild nausea and a feeling of satiety.16Mayo Clinic. What is stevia? I’ve heard it’s good for weight control. Available at: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/expert-answers/stevia/faq-20057856. Raw/crude/whole-leaf stevia are not approved or GRAS due to health concerns.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58. Sucralose is derived from sucrose through chlorination. When combined with bulking agents, sucralose serves as a granular table sweetener.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701.
Consumption of AS
In one survey (2009 to 2012), an estimated 31% of adults consumed ASBs.17Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet 2017;117:441.e2-448. In 2014, an estimated 18.3% of pregnant women in the US consumed ASB, with an overall prevalence of AS consumption of 24%.18Sylvetsky AC, Figueroa J, Rother KI, Goran MI, Welsh JA. Trends in low-calorie sweetener consumption among pregnant women in the United States. Curr Dev Nutr 2019;3(4):nzz004. Acceptable daily intakes (ADIs) are based on body weight. (Table 2) Actual AS intake has been found to be generally well within these limits.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,19Martyn D, Darch M, Roberts A, Lee HY, Tian TY, Kaburagi N, Belmar P. Low-/no-calorie sweeteners: A review of global intakes. Nutrients 2018;10:357. For swingle fruit extract, there is no upper limit.
| Table 2. ADI, equivalent # sweetener packets and cans of soda15United States Food & Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. Available at: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states#SummaryTable.,20http://www.sugar-and-sweetener-guide.com/acceptable-daily-intake.html | |||
|---|---|---|---|
| ADI (mg/kg body weight) | # Packets (equal in sweetness to 2 tsp sugar) for person weighing 60 kg /132 lb. | # Cans 330 ml (~11 fl oz) soda (compared to cans containing 9 tsp sugar) | |
| Acesulfame-K | 15 mg/kg | 23 | 36 |
| Advantame | 32.8 mg/kg | 4,920 | 888 |
| Aspartame | 50 mg/kg | 75 | 37 |
| Neotame | 0.3 mg/kg | 23 | 44 |
| Saccharin | 15 mg/kg | 45 | 56 |
| Refined Stevia* | 4 mg/kg | 9 | 6 |
| Sucralose | 5 mg/kg | 23 | 36 |
*Source: ADI established by the Joint FAO/WHO Expert Committee on Food Additives
AS and Oral Health
AS cannot be metabolized by cariogenic bacteria and are therefore noncariogenic.5Al Humaid J. Sweetener content and cariogenic potential of pediatric oral medications: A literature. Int J Health Sci (Qassim) 2018;12(3):75-82.,13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701.,21Touger-Decker R, van Loveren C. Sugars and dental caries. Am J Clin Nutr 2003;78(4):881S-92S. The FDA first permitted a health claim on noncariogenicity in 1996.22McNutt K. Sugar replacers and the FDA noncariogenicity claim. J Dent Hyg 2000;74(1):36-40. Noncariogenic sweeteners cannot contain sugar, and must maintain a pH ≥5.7 during or up to 30 minutes after consumption.22McNutt K. Sugar replacers and the FDA noncariogenicity claim. J Dent Hyg 2000;74(1):36-40. This health claim was permitted for sucralose in 2006, with studies on bacterial metabolism, experimental caries and in situ plaque pH confirming its noncariogenicity.23Food and Drug Administration, HHS. Food labeling: health claims; dietary noncariogenic carbohydrate sweeteners and dental caries. Final rule. Fed Regist 2006;71(60):15559-64.,24Mandel ID, Grotz VL. Dental considerations in sucralose use. J Clin Dent 2002;13(3):116-8. Notably, bulking agents can be cariogenic.24Mandel ID, Grotz VL. Dental considerations in sucralose use. J Clin Dent 2002;13(3):116-8. Products containing sucralose, or sucralose plus aspartame, are bulked with dextrose and/or maltodextrin. Dextrose is a fermentable carbohydrate, and maltodextrin is a starch with cariogenic potential.21Touger-Decker R, van Loveren C. Sugars and dental caries. Am J Clin Nutr 2003;78(4):881S-92S.,25Rezende G, Hashizume LN. Maltodextrin and dental caries: a literature review. Rev Gaúch Odontol 2018;66(3):257-62. Sucralose is also available in a 50:50 mix with sugar.
AS function as noncariogenic sources of sweetness in dentifrices, typically representing around 0.2% of a dentifrice’s volume. Options include saccharin, acesulfame-K and sucralose. Xylitol and sorbitol, which are nutritive sweeteners, may also be used. Providing a sweet profile encourages use and compliance with routine oral hygiene by improving dentifrice appeal.
AS and Systemic Health
Study results on the impact of AS on systemic health are conflicting. In a 2014 review of prospective cohort studies in children, no clear association was found for weight gain/obesity and ASB, and weak evidence for an association with sugar-sweetened beverages (SSB).26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808. In randomized controlled trials (RCT), no overall association was found for SSB or ASB consumption and weight gain.26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808. In adults, studies variously show an association for ASB with weight loss or weight gain, or no significant weight difference compared to SSB intake.26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808. Intense sweetness is hypothesized to stimulate appetite or promote sucrose dependency.13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701.,26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808.,27Smeets PA, Weijzen P, de Graaf C, Viergever MA. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage 2011;54(2):1367-74. Several studies have reported no changes in appetite occurred in individuals consuming AS or receiving AS as a pre-load.28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264.,29Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr 2011;65(4):508-13.,30Maersk M, Belza A, Holst JJ, Fenger-Gron M, Pedersen SB, Astrup A, Richelsen B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. Eur J Clin Nutr 2012;66(4):523-9.,31Hall WL, Millward DJ, Rogers PJ, Morgan LM. Physiological mechanisms mediating aspartame induced satiety. Physiol & Behavior 2003;78(4-5):557-62.,32Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010;55(1):37-43.,33Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr 2018;148(4):650-7. For DM, one meta-analysis found a relative risk of 1.13 with intake of 330 ml/day ASB and greater risk with SSB intake. No association with DM was found in a second meta-analysis.28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264. An association for ASB and metabolic disease was, however, found in >50% of observational studies.28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264. In longitudinal studies with approximately 40,000, >340,000 and >70,000 individuals, respectively, no association for ASB and risk for DM was found after adjusting for BMI in one study and for BMI and energy intake in 2 other studies.28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264. Overall, reviewed studies were heterogeneous, and no association for AS and glucose metabolism could be determined.28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264.
A review of databases spanning 1986-2014 and 1980-2014 with >37,000 male and >80,000 female health professionals, who were healthy at baseline, was conducted to determine all-cause and cause-specific mortality.34Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019;139:2113-25. At SSB consumption levels of 1 to 4/month, 2 to 6/week, and >2/day, the increased risk of all-cause mortality compared to <1/month was 1%, 6% and 21%, respectively. In contrast, reduced risk was observed for ASBs except for consumption of >2/day, for which a 4% increased risk was found. (Figure 2) A 16% increase in cancer mortality was associated with SSB consumption, with no increase for ASB consumption.34Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019;139:2113-25. Recent meta-analyses found no differences in risk for bladder or lower urinary tract cancer across 8 case control studies (n=4509).35Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Br Med J 2019;364:k4718. doi: https://doi.org/10.1136/bmj. There is no clear evidence of an association for AS and cancer in humans.36National Cancer Institute. Artificial sweeteners and cancer. Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet.
In a large prospective cohort study, an elevated risk for all stroke, coronary heart disease, and all-cause mortality was found for women consuming ≥2 ASB daily (≥24 ounces/day) compared with those consuming <1 ASB/week.37Mossavar-Rahmani Y, Kamensky V, Manson JE, Silver B, Rapp SR, Haring B, Beresford SAA, Snetselaar L, Wassertheil-Smoller S. Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women’s Health Initiative. Stroke 2019;50:555-62. However, an association for high ASB consumption and stroke was found only for obese individuals, while all-cause mortality increased for individuals of normal weight/overweight.38Gardener H, Elkind MSV. Artificial sweeteners, real risks. Stroke 2019;50:549-51. A limited number of RCT have been conducted on ASB and CVD risk, largely involving ASB as substitutes for SSB.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40. In a review, a 31% and 13% increase in CVD-mortality was found for daily consumption of >2 SSB or ASB, respectively.35Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Br Med J 2019;364:k4718. doi: https://doi.org/10.1136/bmj.
Considerations and Current Recommendations
At an international consensus meeting, it was concluded that AS have been extensively evaluated and their safety confirmed by multiple global regulatory bodies.39Serra-Majem L, Raposo A, Aranceta-Bartrina J, Varela-Moreiras G, Logue C, Laviada H, Socolovsky S, et al. Ibero–American consensus on low- and no-calorie sweeteners: Safety, nutritional aspects and benefits in food and beverages. Nutrients 2018;10,:818. doi:10.3390/nu10070818. However, data on the benefits and risks of ASB consumption specifically is reported to be limited, and there is a lack of data on individual AS.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40. Further, study outcomes on risk for disease are conflicting. Existing studies are heterogeneous for methodology, sample size, duration, scope, and identification/consideration of confounding factors, reverse causality and other biases.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40.,26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808.,28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264.,35Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Br Med J 2019;364:k4718. doi: https://doi.org/10.1136/bmj.
In contrast, a large body of evidence exists on the negative health impact of added sugars in foods and SSB. Two recent guidelines recommended that added sugars be confined to <10% of calories ingested and encouraged a move to healthier foods and beverages.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40. Several organizations have provided statements on ASB and AS. Short-term consumption of ASB and other low-calorie beverages, instead of SSB, may help to reduce energy intake and weight in some adults.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40. ASB and AS may favor weight reduction in structured diet plans (i.e., without compensatory energy intake), and help with glycemic control in DM management.4Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112(5):739-58.,9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40.,26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808.,28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264.,39Serra-Majem L, Raposo A, Aranceta-Bartrina J, Varela-Moreiras G, Logue C, Laviada H, Socolovsky S, et al. Ibero–American consensus on low- and no-calorie sweeteners: Safety, nutritional aspects and benefits in food and beverages. Nutrients 2018;10,:818. doi:10.3390/nu10070818. As noted in one study, ASB consumption should not be encouraged, while ASB and AS may be of value for the management of metabolic diseases.28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264. As there is little long-term data for ASB intake by children, long-term ASB intake should be avoided in children.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40.
Conclusions
Future research on AS and ASB is recommended, using a standardized methodology, and long-term prospective epidemiological studies.19Martyn D, Darch M, Roberts A, Lee HY, Tian TY, Kaburagi N, Belmar P. Low-/no-calorie sweeteners: A review of global intakes. Nutrients 2018;10:357.,26Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk 1–3. Adv Nutr 2014;5:797-808.,28Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016;11(8):e0161264.,35Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Br Med J 2019;364:k4718. doi: https://doi.org/10.1136/bmj. This would define the potential uses, benefits and risks of AS, and ASB consumption in particular, including for conditions with few or no studies, and thereby enhance patient-level recommendations and risk management.19Martyn D, Darch M, Roberts A, Lee HY, Tian TY, Kaburagi N, Belmar P. Low-/no-calorie sweeteners: A review of global intakes. Nutrients 2018;10:357.,35Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Br Med J 2019;364:k4718. doi: https://doi.org/10.1136/bmj. Research on the safety of AS during pregnancy, childhood and adolescence with respect to longer-term health outcomes is also recommended.9Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126-40.,18Sylvetsky AC, Figueroa J, Rother KI, Goran MI, Welsh JA. Trends in low-calorie sweetener consumption among pregnant women in the United States. Curr Dev Nutr 2019;3(4):nzz004.,35Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Br Med J 2019;364:k4718. doi: https://doi.org/10.1136/bmj.
Dental professionals can advise patients and parents on weight and caries control.13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701. Individualized evaluation and advice should be provided based on current recommendations, considering the patient’s age, risk for oral and systemic disease, and suitability of AS as part of a strategy to reduce sugar and energy intake. Patients should also be educated on the need to limit children’s consumption of low-pH foods and beverages (SSB and ASB) due to the risk of dental erosion, as well as intake of foods with high levels of natural and added sugars and processed starches to reduce the risk of dental caries.13Roberts MW, Wright JT. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 2012;Article ID 625701.,40American Dental Association. Policies and recommendations on diet and nutrition. Available at: https://www.ada.org/en/advocacy/current-policies/diet-and-nutrition. Further, patients can be reassured of the safety of AS as sweeteners in dentifrice formulations. Consumer education on products containing AS and continued education for health professionals have been recommended.39Serra-Majem L, Raposo A, Aranceta-Bartrina J, Varela-Moreiras G, Logue C, Laviada H, Socolovsky S, et al. Ibero–American consensus on low- and no-calorie sweeteners: Safety, nutritional aspects and benefits in food and beverages. Nutrients 2018;10,:818. doi:10.3390/nu10070818. In addition, position statements and consensus documents would be beneficial for healthcare professionals.39Serra-Majem L, Raposo A, Aranceta-Bartrina J, Varela-Moreiras G, Logue C, Laviada H, Socolovsky S, et al. Ibero–American consensus on low- and no-calorie sweeteners: Safety, nutritional aspects and benefits in food and beverages. Nutrients 2018;10,:818. doi:10.3390/nu10070818. Dental and other healthcare professionals should keep apprised of developments in nutrition and the use of AS.
References
- 1.Zimmerli B, Jeger F, Lussi A. Bleaching of nonvital teeth. A clinically relevant literature review. Schweiz Monatsschr Zahnmed 2010;120(4):306-20.
- 2.Brunton PA, Burke FJT, Sharif MO et al. Contemporary dental practice in the UK in 2008: Aspects of direct restorations, endodontics, and bleaching. Br Dent J 2012;212:63-7.
- 3.Demarco FF, Conde MCM, Ely C et al. Preferences on vital and nonvital tooth bleaching: A survey among dentists from a city of Southern Brazil. Braz Dent J 2013;24:527-31.
- 4.Kahler B. Present status and future directions – Managing discoloured teeth. Int Endod J 2022: Feb 21. doi: 10.1111/iej.13711.
- 5.Nutting EB, Poe GS. Chemical bleaching of discolored endodontically treated teeth. Dent Clin N Am 1967;11:655-62.
- 6.Pallarés-Serrano A, Pallarés-Serrano S, Pallarés-Serrano A, Pallarés-Sabater A. Assessment of Oxygen Expansion during Internal Bleaching with Enamel and Dentin: A Comparative In Vitro Study. Dent J 2021;9:98. https://doi.org/10.3390/dj9090098.
- 7.Estay J, Angel P, Bersezio C et al. The change of teeth color, whiteness variations and its psychosocial and self-perception effects when using low vs. high concentration bleaching gels: a one-year follow-up. BMC Oral Health 2020;20(1):255. doi: 10.1186/s12903-020-01244-x.
- 8.Haywood VB, Bergeron BE. Bleaching and the Diagnosis of Internal Resorption. Jul 24, 2018. https://decisionsindentistry.com/article/bleaching-and-the-diagnosis-of-internal-resorption/
- 9.Abbott P, Heah SY. Internal bleaching of teeth: an analysis of 255 teeth. Aust Dent J 2009;54(4):326-33. https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1834-7819.2009.01158.x.
- 10.Sakalli B, Basmaci F, Dalmizrak O. Evaluation of the penetration of intracoronal bleaching agents into the cervical region using different intraorifice barriers. BMC Oral Health 2022;22(1):266. doi: 10.1186/s12903-022-02300-4.
- 11.American Association of Endodontists Clinical Practice Committee. Use of Silver Points. AAE Position Statement. https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/silverpointsstatement.pdf#:~:text=Silver%20points%20have%20been%20shown%20to%20corrode%20spontaneously,retreatment%20and%20replacement%20of%20the%20points%20with%20another.
- 12.Savadkouhi ST, Fazlyab M. Discoloration Potential of Endodontic Sealers: A Brief Review. Iran Endod J 2016;11(4):250-4. doi: 10.22037/iej.2016.20.
- 13.Ahmed HM, Abbott PV. Discolouration potential of endodontic procedures and materials: a review. Int Endod J 2012;45(10):883-97. doi: 10.1111/j.1365-2591.2012.02071.x.
- 14.Krastl G, Allgayer N, Lenherr P et al. Tooth discoloration induced by endodontic materials: a literature review. Dent Traumatol 2013;29(1):2-7. doi: 10.1111/j.1600-9657.2012.01141.x.
- 15.Ioannidis K, Mistakidis I, Beltes P, Karagiannis V. Spectrophotometric analysis of crown discoloration induced by MTA- and ZnOE-based sealers. J Appl Oral Sci 2013;21(2):138-44. doi: 10.1590/1678-7757201302254.
- 16.Santos LGPD, Chisini LA, Springmann CG et al. Alternative to Avoid Tooth Discoloration after Regenerative Endodontic Procedure: A Systematic Review. Braz Dent J 2018;29(5):409-18. doi: 10.1590/0103-6440201802132.
- 17.Watts A, Addy M. Tooth discoloration and staining: a review of the literature. Br Dent J 2001;190:309-16.
- 18.Pink tooth of Mummery. https://medical-dictionary.thefreedictionary.com/Pink+Tooth+of+Mummery.
- 19.Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int 1992:23:471-88. http://www.quintpub.com/userhome/qi/qi_23_7_haywood_6.pdf
- 20.Bersezio C, Ledezma P, Mayer C et al. Effectiveness and effect of non-vital bleaching on the quality of life of patients up to 6 months post-treatment: a randomized clinical trial. Clin Oral Investig 2018;22(9):3013-9. doi: 10.1007/s00784-018-2389-y.
- 21.Popescu AD, Purcarea MV, Georgescu RV et al. Vital and Non-Vital Tooth Bleaching Procedures: A Survey Among Dentists From Romania. Rom J Oral Rehab 2021;13(3):59-71. https://www.rjor.ro/wp-content/uploads/2021/10/VITAL-AND-NON-VITAL-TOOTH-BLEACHING-PROCEDURES-A-SURVEY-AMONG-DENTISTS-FROM-ROMANIA.pdf.
- 22.Zarean P, Zarean P, Ravaghi A et al. Comparison of MTA, CEM Cement, and Biodentine as Coronal Plug during Internal Bleaching: An In Vitro Study. Int J Dent 2020;2020:8896740. doi: 10.1155/2020/8896740.
- 23.Canoglu E, Gulsahi K, Sahin C et al. Effect of bleaching agents on sealing properties of different intraorifice barriers and root filling materials. Med Oral Patol Oral Cir Bucal 2012;17 (4):e710-5. http://www.medicinaoral.com/medoralfree01/v17i4/medoralv17i4p710.pdf
- 24.Amato A, Caggiano M, Pantaleo G, Amato M. In-office and walking bleach dental treatments on endodontically-treated teeth: 25 years follow-up. Minerva Stomatol 2018;67(6):225-30.
- 25.Lim MY, Lum SOY, Poh RSC et al. An in vitro comparison of the bleaching efficacy of 35% carbamide peroxide with established intracoronal bleaching agents. Int Endod J 2004;37(7):483-8. doi: 10.1111/j.1365-2591.2004.00829.x.
- 26.Yui KCK, Rodrigues JR, Mancini MNG et al. Ex vivo evaluation of the effectiveness of bleaching agents on the shade alteration of blood-stained teeth. Int Endod J 2008;41(6):485-92. doi: 10.1111/j.1365-2591.2008.01379.x.
- 27.Warren MA, Wong M, Ingram TA III. An in vitro comparison of bleaching agents on the crowns and roots of discolored teeth. J Endod 1990;16:463-7.
- 28.Rotstein I, Mor C, Friedman S. Prognosis of intracoronal bleaching with sodium perborate preparations in vitro: 1-year study. J Endod 1993;19:10-2.
- 29.Weiger R, Kuhn A, Lost C. In vitro comparison of various types of sodium perborate used for intracoronal bleaching of discolored teeth. J Endod 1994;20:338-41.
- 30.Amato M, Scaravilli MS, Farella M, Riccitiello F. Bleaching teeth treated endodontically: long-term evaluation of a case series. J Endod 2006;32:376-8.
- 31.Abou-Rass M. Long-term prognosis of intentional endodontics and internal bleaching of tetracycline-stained teeth. Compend Contin Educ Dent 1998;19:1034-44.
- 32.Anitua E, Zabalegui B, Gil J, Gascon F. Internal bleaching of severe tetracycline discolorations: four-year clinical evaluation. Quintessence Int 1990;21(10):783-8.
- 33.Gorr S-U, Brigman HV, Anderson JC, Hirsch EB. The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS ONE 2022;17(8): e0273504. https://doi.org/10.1371/journal.pone.0273504.
- 34.Peters SM, Hill NB, Halepas S. Oral manifestations of monkeypox: A report of two cases Journal of Oral and Maxillofacial Surgery (2022). https://doi.org/10.1016/j.joms.2022.07.147.
- 35.Portalatin A. Infectious disease experts call on dentists to monitor monkeypox symptoms: 6 notes. August 15, 2022. https://www.beckersdental.com/clinical-leadership-infection-control/39124-infectious-disease-experts-call-on-dentists-to-monitor-monkeypox-symptoms-6-notes.html?origin=DentalE&utm_source=DentalE&utm_medium=email&utm_content=newsletter&oly_enc_id=1694C1316967A3F.
- 36.CDC. Monkeypox. Non-Variola Orthopoxvirus and Monkeypox Virus Laboratory Testing Data. August 17, 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/2022-lab-test.html.
- 37.CDC. Monkeypox. Prevention. https://www.cdc.gov/poxvirus/monkeypox/prevention.html.
- 38.CDC. Monkeypox and smallpox vaccine guidance. Updated June 2, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/index.htm.
- 39.CDC. Monkeypox. ACAM Vaccine. https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/acam2000-vaccine.html.
- 40.CDC. Guidelines for Infection Control in Dental Health-Care Settings — 2003. https://www.cdc.gov/mmwr/PDF/rr/rr5217.pdf.
- 41.CDC. Infection Prevention and Control of Monkeypox in Healthcare Settings. Updated August 11, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- 42.CDC. Monkeypox. Information For Veterinarians. https://www.cdc.gov/poxvirus/monkeypox/veterinarian/index.html.
- 43.CDC. Monkeypox. Pets in the Home. https://www.cdc.gov/poxvirus/monkeypox/prevention/pets-in-homes.html.
- 44.Cheng YL, Musonda J, Cheng H, et al. Effect of surface removal following bleaching on the bond strength of enamel. BMC Oral Health 2019;19(1):50.
- 45.Monteiro D, Moreira A, Cornacchia T, Magalhães C. Evaluation of the effect of different enamel surface treatments and waiting times on the staining prevention after bleaching. J Clin Exp Dent 2017;9(5):e677-81.
- 46.Rezende M, Kapuchczinski AC, Vochikovski L, et al. Staining power of natural and artificial dyes after at-home dental bleaching. J Contemp Dent Pract 2019;20(4):424-7.
- 47.Mickenautsch S, Yengopal V. Effect of xylitol versus sorbitol: a quantitative systematic review of clinical trials. Int Dent J 2012;62(4):175-88.
- 48.Rethman MP, Beltrán-Aguilar ED, Billings RJ, Burne RA, Clark M, Donly KJ, Hujoel PP, Katz BP, Milgrom P, Sohn W, Stamm JW, Watson G, Wolff M, Wright T, Zero D, Aravamudhan K, Frantsve-Hawley J, Meyer DM; for the American Dental Association Council on Scientific Affairs Expert Panel on Nonfluoride Caries-Preventive Agents. Nonfluoride caries-preventive agents. Executive summary of evidence-based clinical recommendations. J Am Dent Assoc 2011;142(9):1065-71.
- 49.Milgrom P, Söderling EM, Nelson S, Chi DL, Nakai Y. Clinical evidence for polyol efficacy. Adv Dent Res 2012; 24(2):112-6.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2019/07/Artificial-Sweeteners-2.jpg)