Maintaining and monitoring dental unit waterlines (DUWL) in accordance with current recommendations is an essential component of safe dental visits. DUWL deliver procedural water to handpieces, ultrasonic scalers, and other devices connected to the waterlines. In the United States, the acceptable microbiological quality of procedural water must be ≤500 CFU/ml of heterotrophic water bacteria which meets the Environmental Protection Agency’s regulatory requirements for safe drinking water,1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.,2U.S. Environmental Protection Agency, National Primary Drinking Water Regulations, 1999 Available at: http://www.epa.gov/OGWDW/wot/appa.html while achieving lower levels is desirable. Further, DUWL-sourced procedural water is not appropriate for surgical procedures, and during a ‘boil water advisory’ municipal water should never be run through the dental unit nor used for any patient care.1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.
How procedural water is contaminated
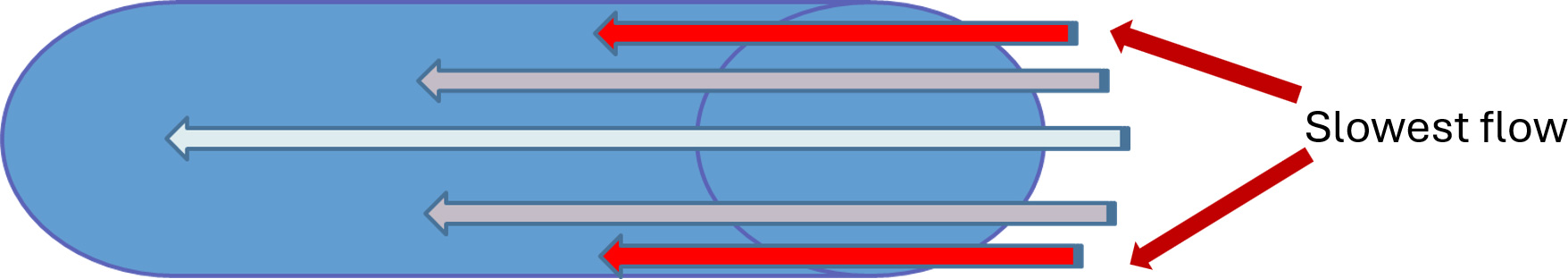
Since the EPA regulatory requirement is ≤500 CFU/ml of heterotrophic water bacteria for safe drinking water, it would be easy to think that procedural water would be inherently safe for use when sourced from municipal water. That, of course, is not the case. Within 5 days of installation of new DUWL, microbial counts can reach 200,000 colony-forming units (CFU)/mL,3Barbeau J, Tanguay R, Faucher E et al. Multiparametric Analysis of Waterline Contamination in Dental Units. Amer Soc Microbiol 1996;62(11):3954-9. and for untreated DUWL, microbial contamination in procedural water may exceed 1,000,000 CFU/mL.1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf. DUWL consist of extensive tubing with narrow lumens that are 2 to 3 mm in diameter and a large surface area relative to volume.4Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. In: Molinari JA, Harte JA, eds. Cottone’s practical infection control in dentistry. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams; 2005:63-75.,5Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106(5):1424-37. doi: 10.1111/j.1365-2672.2008.04100.x. The laminar flow of water within the tubing means that the water adjacent to the inner walls of the tubing moves more slowly than in the middle of the lumen, and at the interface of the lumen and water flow (the hydrodynamic layer) the water is stagnant.4Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. In: Molinari JA, Harte JA, eds. Cottone’s practical infection control in dentistry. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams; 2005:63-75.,5Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106(5):1424-37. doi: 10.1111/j.1365-2672.2008.04100.x.,6O’Donnell MJ, Boyle MA, Russell RJ, Coleman DC. Management of dental unit waterline biofilms in the 21st century. Future Microbiol 2011;6(10):1209-26. doi: 10.2217/fmb.11.104. (Figure 1) Extracellular polysaccharides secreted by microorganisms act as a glue, holding colonizers and the biofilm mass together.
These characteristics, along with the tubing material, result in the deposition of minerals from the water onto the tubing which encourages microbial adhesion, colonization, and rapid biofilm formation.4Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. In: Molinari JA, Harte JA, eds. Cottone’s practical infection control in dentistry. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams; 2005:63-75.,5Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106(5):1424-37. doi: 10.1111/j.1365-2672.2008.04100.x. DUWL are also used intermittently during workdays and regularly dormant for extended periods (e.g., at night and weekends), further encouraging biofilm formation and growth. In addition, while anti-retraction valves are now routinely incorporated into dental devices, if these are not properly maintained suck-back of oral fluids into DUWL can occur. Microorganisms in the DUWL are shed and then carried to the oral environment in procedural water, where they are also aerosolized during aerosol-generating procedures and may be inhaled and settle on environmental surfaces.
Microbial contaminants in DUWL
DUWL contaminants comprise a wide range of microorganisms, including opportunistic pathogens and pathogens.7Pankhurst CL, Scully C, Samaranayake L. Dental Unit Water Lines and their Disinfection and Management: A Review. Dent Update 2017;44(4):284-5, 289-92. doi: 10.12968/denu.2017.44.4.284. In a recent systematic review and meta-analysis (2023), it was concluded that 12% and 8% of DUWL-derived (opportunistic) pathogens, respectively, were Pseudomonas aeruginosa and Legionella pneumonophila.8Bayani M, Raisolvaezin K, Almasi-Hashiani A, Mirhoseini SH. Bacterial biofilm prevalence in dental unit waterlines: a systematic review and meta-analysis. BMC Oral Health 2023;23 (1):158.
In addition to Pseudomonas aeruginosa and Legionella sp., an extensive range of microorganisms is found in DUWL, including nontuberculous mycobacteria (NTM), Candida species, Actinomyces sp., Bacillus sp., Lactobacillus sp., Staphylococcus aureus, Streptococcus sp., Bacteroides, Acinetobacter sp., Burkholderia cepacian, Fusobacteria, Klebsiella pneumoniae, Salmonella sp., Aspergillus, Escheria coli, Shigella, Giardia and other protozoa.1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.,9Barbot V, Robert A, Rodier M-H, Imbert C. Update on infectious risks associated with dental unit waterlines. FEMS Immunol Med Microbiol 2012;65(2):196-204.,10Tuvo B, Totaro M, Cristina ML et al. Prevention and control of legionella and Pseudomonas spp. colonization in dental units. Pathogens 2020;9(4):1-12.,11Ditommaso S, Giacomuzzi M, Ricciardi E et al. Colonization by Pseudomonas aeruginosa of dental unit water lines and its relationship with other bacteria: suggestions for microbiological monitoring. J Water Health 2019;17(4):532-9.
The evidence base for DUWL monitoring and maintenance
A Health Alert Network (HAN) for dental settings issued by the Centers for Disease Control and Prevention in October 2022 was precipitated by a third outbreak of NTM infections in children receiving pulpotomies during which procedural water was used. Prior NTM outbreaks occurred in a pediatric dental clinic in Georgia (2015), which affected 20 children, and in a clinic in California (2016) which resulted in NTM infections in 71 pediatric dental patients, 70 of whom required hospitalization.12Peralta G, Tobin-D’Angelo M, Parham A et al. Notes from the Field: Mycobacterium abscessus Infections Among Patients of a Pediatric Dentistry Practice--Georgia, 2015. Morb Mortal Wkly Rep 2016;65(13):355-6.,13Singh J, O’Donnell K, Nieves DJ et al. Invasive Mycobacterium abscessus Outbreak at a Pediatric Dental Clinic. Open Forum Infect Dis 2021;8(6):165. https://doi.org/10.1093/ofid/ofab165. Complications included lymphadenopathy, permanent tooth loss, significant bone loss, facial nerve palsy, and incision fibrosis. In 32 of the 70 hospitalized children in California, intravenous antibiotic therapy was needed for extended periods of time (median duration 137 days), with some patients consequently experiencing hearing loss.
In earlier incidents, two deaths were associated with Legionella sp. following exposure to contaminated procedural water during dental treatment, the first an elderly immune-compromised Swedish patient in 2012, and the second one an elderly Italian patient in 2014.14Schönning C, Jernberg C, Klingenberg D et al. Legionellosis acquired through a dental unit: a case study. J Hosp Infect 2017;96(1):89-92. https://doi.org/10.1016/j.jhin.2017.01.009.,15Ricci ML, Fontana S, Pinci F et al. Pneumonia associated with a dental unit waterline. The Lancet 2012;379(9816):684. In addition, in two immunocompromised patients, oral abscesses resulted from exposure during dental treatment to pseudomonas which was confirmed by testing to match the strain in the DUWL.16Martin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J 1987; 163:152-4. Of note, Pseudomonas aeruginosa is multi-drug resistant and a leading cause of healthcare-acquired infections in the US.17Bassetti M, Vena A, Croxatto A et al. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018;7:212527. doi: 10.7573/dic.212527.
Procedural water and aerosol-generating procedures
DUWL microbial contaminants contribute significantly to procedure-generated aerosols, along with microorganisms from the oral environment.5Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106(5):1424-37. doi: 10.1111/j.1365-2672.2008.04100.x.,18Allison JR, Dowson C, Jakubovics NS et al. Waterline Disinfectants Reduce Dental Bioaerosols: A Multitracer Validation. J Dent Res 2022;101(10):1198-204. doi: 10.1177/00220345221093522. ,19Poolkerd W, Swatasuk B, Saengpitak M et al. Metataxonomics study of dental bioaerosols affected by waterline disinfection. BMC Oral Health 2024;24(1):1575. doi: 10.1186/s12903-024-05304-4. ,20Dutil S, Veillette M, Mériaux A et al. Aerosolization of mycobacteria and legionellae during dental treatment: low exposure despite dental unit contamination. Environ Microbiol 2007;9(11):2836-43.,21Zemouri C, Volgenant CMC, Buijs MJ et al. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol 2020;12(1):1762040. In one study, individual patients received aerosol-generating procedures in one of two dental units in separate operatories.19Poolkerd W, Swatasuk B, Saengpitak M et al. Metataxonomics study of dental bioaerosols affected by waterline disinfection. BMC Oral Health 2024;24(1):1575. doi: 10.1186/s12903-024-05304-4. Air, saliva and procedural samples were obtained, and it was determined that 18.6% of aerosolized bacteria originated from contaminated procedural water. In another study, passive sampling methods were used before, during and after treatment and at various locations in four dental clinics.21Zemouri C, Volgenant CMC, Buijs MJ et al. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol 2020;12(1):1762040. Although the overall level of contamination was considered low, the researchers reported that 36 of 98 microbial taxa aerosolized were from procedural water.
While Legionellosis has not been reported in DHCP, studies have indicated significantly higher titers of antibodies to Legionella sp., with 23% of DHCP in one study having IgG antibodies to Legionella compared to 8% in the general population in the same community.22Fotos PG, Westfall HN, Snyder IS et al. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J Dent Res 1985;64(12):1382–5. In contrast, in a more recent study conducted with more than 5,000 US dentists between 2002 and 2012, no significant differences were found for the prevalence of antibodies to L. pneumophila compared to the general population.23Estrich CG, Gruninger SE, Lipman RD. Rates and predictors of exposure to Legionella pneumophila in the United States among dental practitioners: 2002 through 2012. J Am Dent Assoc 2017;148(3):164-71. This may have been the result of changes in infection prevention protocols over time. Aerosolized bacterial endotoxins are a further contaminant in procedural water,5Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106(5):1424-37. doi: 10.1111/j.1365-2672.2008.04100.x.,24Szymanska J. Exposure to bacterial endotoxin during conservative dental treatment. Ann Agric Environ Med 2005;12(1):137-9. and found to be a potential risk for occupationally acquired asthma.25Pankhurst CL, Coulter W, Philpott-Howard JN et al. Evaluation of the potential risk of occupational asthma in dentists exposed to contaminated dental unit waterlines. Prim Dent Care 2005;12(2):53-9. It is recognized that contaminated DUWL pose a risk for infection in patients as a result of direct exposure via procedural water, and a risk for infection among DHCP due to inhalation of aerosols generated during procedures.26U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines Taken together, the above facts highlight the essential nature of robust protocols for the appropriate management of DUWL.
Factors influencing the microbial quality of procedural water include the adequacy of DUWL monitoring and maintenance (frequency and method), adherence to manufacturers’ and laboratory test IFUs, and staff training and compliance.1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.,26U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines,27Ji XY, Fei CN, Zhang Y et al. Three key factors influencing the bacterial contamination of dental unit waterlines: a 6-year survey from 2012 to 2017. Int Dent J 2019;69(3):192-9. doi: 10.1111/idj.12456.,28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27. Engineering flaws, that must be avoided and corrected include design issues such as dead legs in waterlines which harbor microbial contamination, and leakage (e.g., from suction hoses).27Ji XY, Fei CN, Zhang Y et al. Three key factors influencing the bacterial contamination of dental unit waterlines: a 6-year survey from 2012 to 2017. Int Dent J 2019;69(3):192-9. doi: 10.1111/idj.12456.,29O’Donnell MJ, Tuttlebee CM, Falkiner FR, Coleman DC. Bacterial contamination of dental chair units in a modern dental hospital caused by leakage from suction system hoses containing extensive biofilm. J Hosp Infect 2005;59(4):348-60. (Table 1) DUWL should be monitored (inspected) for damage, and if present replaced or managed in accordance with the manufacturer’s IFU.26U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines
| Table 1. Factors influencing the microbial quality of procedural water |
|---|
| Source water |
| Adherence to manufacturers’ IFU for DUWL monitoring products, maintenance products and the dental unit |
| Adherence to laboratory IFU (DUWL monitoring) |
| Adequacy of DUWL monitoring and maintenance |
| Staff training and compliance |
| Functionality of anti-retraction valves |
| DUWL design issues such as dead legs |
| Leakage from suction hoses |
| Other engineering flaws |
Management of DUWL
Flushing, monitoring, and maintaining DUWL is essential. DUWL should be flushed for 20 to 30 seconds at the beginning and end of the day and between patients, where handpieces, scalers, air-water syringes, and other devices were connected to the DUWL during patient care. Flushing may help to reduce the microbial load; it does not eliminate biofilm and is insufficient to provide quality procedural water.1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.,28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27.,30Lizzadro J, Mazzotta M, Girolamini L et al. Comparison between two types of dental unit waterlines: how evaluation of microbiological contamination can support risk containment. Int J Environ Res Public Health 2019;16(3):1-14. In addition to the measures described below, DHPC should ‘be alert to signs that may indicate biofilm formation including musty odor, cloudiness or particulates in the water, and clogging of lines’.26U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines
DUWL monitoring (testing)
Monthly DUWL monitoring is recommended for newly installed DUWL treatment devices; following changes to the manufacturer’s IFU; the adoption of new protocols or DUWL treatment products; after repairing dental units or devices; and, when DUWL have not been used for an extended time or were inadequately/not maintained.28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27. This frequency can be reduced to at a minimum every three months if prior testing results were acceptable in two adjacent months of testing. Routine testing at least every three months is recommended for ongoing monitoring of DUWL that meet the acceptable microbial quality for procedural water. Note that if requirements in your location dictate more frequent testing, this should be followed. Additionally, DUWL testing more frequently than quarterly and/or screening between quarterly tests may provide peace of mind and can function as an early warning system for potential issues.
DUWL Test options
Testing options include laboratory and in-office tests. Regardless of which type of test is being used, the manufacturer’s written IFU (or laboratory IFU) should be followed. If monitoring shows that procedural water does not meet the required quality, remedial action must be taken. State rules/acts related to DUWL may specify testing frequency, which tests are acceptable, and whether documentation and reports must be obtained from an independent testing entity (i.e., requiring a laboratory test). DHCP should check for these and other mandated requirements and follow the testing method(s) and frequency of testing acceptable in their location.
Laboratory tests provide third-party verification. The Reasoner’s 2 Agar test (R2A) is recommended, has a 5-to-7-day incubation period, and is more accurate than in-office testing.28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27. It provides a suitable baseline measure (i.e., before making changes to DUWL maintenance protocols). The membrane filtration method or a test equivalent to R2A is also suggested. Flow cytometry is a newer test that uses laser technology to obtain a bacterial count (no incubation period), and this technology can also identify the types of bacteria by using dyes which is otherwise difficult and not performed in other DUWL laboratory tests.
In-office testing using the heterotrophic plate count method (HPC) is recommended.28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27. Paddle-type devices use this method, and consist of a vial for the sample and a paddle with a grid on which viable bacteria are visible for counting after incubation (typically 48 to 72 hours at room temperature). (Figure 2) Fifteen-minute in-office screening tests are now also available, do not require incubation, and provide a simple pass/fail result based on whether the water sample exceeds 500 CFU/ml of heterotrophic bacteria. Screening may assist in the management of DUWL by providing additional information between routine testing.
Sample collection
Sample collection should follow the manufacturer’s IFU/laboratory IFU. General sampling steps are as follows:
- Detach any used dental devices connected to the DUWL.
- Flush the DUWL (each line) for 20 to 30 seconds.
- Collect the sample(s) directly or through sterilized dental devices attached to the unit
- Separate samples from each of the DUWL outlets, or a pooled sample – if a pooled sample is obtained, ensure that equal amounts are obtained from each DUWL outlet.
- If required (see manufacturer’s IFU), perform germicidal neutralization
- Include labeling for the sample specifying the sources (unit, handpiece, air-water syringe etc.) and date/time collected.
Failure to appropriately handle test materials will result in inaccuracies in results. Samples need to be collected aseptically, hand hygiene performed and gloves donned prior to sampling, to avoid contamination (including skin squames) and to protect DHCP.
Maintaining (Treating) DUWL
The manufacturer’s written IFU for the dental unit or the waterline product should be followed, and the infection control coordinator should contact the manufacturer if necessary to obtain supplemental information. Additionally, shock treatment with a high dose of an appropriate germicidal agent is recommended first if the dental unit has not been tested or properly maintained, in accordance with the treatment product manufacturer’s IFU. Further remedial action may be required based on the subsequent test.
Options for the maintenance of DUWL include in-line cartridges, independent water bottle systems with germicide-releasing straws, and chemical treatments, self-contained water systems, and central water systems. In-line cartridges connect directly to the municipal water supply in the junction box at the dental chair and provide continuous delivery of the germicidal agent. A second option is a germicidal straw placed in an independent water reservoir bottle containing potable water chairside, also providing for continuous disinfection. The straw is attached to the uptake tube and sits inside the bottle, allowing water to continuously filter through it. Independent water reservoir bottles should be drained of water at the end of the workday. When handling reservoir bottles, hand hygiene should be performed and gloves donned to prevent contamination. Available options include cartridges and straws with either elemental iodine or silver. Cartridges and straws with slow release of germicidal agents are available for 365 days of use; note that if water throughput exceeds the amount stated in the IFU for that cartridge or straw, the product should be changed out to ensure adequate continuous disinfection. (Table 2)
| Table 2. Options for DUWL maintenance |
|---|
| In-line cartridges – Continuous disinfection |
| Independent reservoir water bottle with straws – Continuous disinfection |
| Germicidal tablets and solutions – Continuous or intermittent |
| Central water systems – Filtration device (must also contain a germicidal agent for DUWL maintenance) |
| Self-contained water systems |
Chemical treatments for the continuous treatment of independent water bottles are also available as tablets and solutions. Intermittent treatment products are also available as solutions and tablets. Central water systems are connected to the municipal water source and can remove minerals, salts, and chlorine before the water enters DUWL. To shock and maintain DUWL, the central system must also release a suitable antimicrobial agent. With respect to microfiltration devices, while these may remove organisms from incoming water, they have no effect on biofilm adhered to DUWL. (Table 2)
Documentation and Recordkeeping
DUWL monitoring and maintenance must be documented and retained in the records for the time specified by state and federal requirements (e.g., Georgia’s new Rule specifies 5 years31Georgia Board of Dentistry. Georgia Board of Dentistry Adopts Rule on Dental Unit Water Quality, August 29, 2025. https://gbd.georgia.gov/press-releases/2025-08-29/georgia-board-dentistry-adopts-rule-dental-unit-water-quality. ). The test method, test date, specific dental unit, source and sampling method (sampled lines if pooled), test results, and DHCP performing sampling must be documented. For tests that failed, the method used for remediation and the results of testing after remediation should also be documented, as well as the temporary or permanent removal from service of any dental unit. DUWL maintenance must also be fully documented, as must staff training.
Sterile Water Delivery Systems
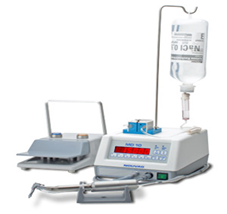
During oral surgery procedures, sterile solutions (typically sterile saline or sterile water) are essential for irrigation and as a coolant. Sterile solutions may be delivered using a bulb syringe, or single-use disposable sterile devices. Stand-alone handpiece/scaler systems (i.e., do not attach to the dental unit) are a further option, with sterile tubing that is either single-use disposable or that can be sterilized after use in accordance with the manufacturer’s written IFU.1Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.,26U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines,28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27. (Figure 3) These are independent of the dental unit water supply.
Of note, NTM infections have occurred when contaminated procedural water was used during surgical procedures. Three cases were reported in 2020 involving otherwise healthy dental patients ages 13, 18 and 21 years in Quito, Ecuador and Caracas, Venezuela. Two patients had undergone third molar extractions and the youngest received treatment for an abscessed first molar.32Pérez-Alfonzo R, Poleo Brito LE, Vergara MS et al. Odontogenic cutaneous sinus tracts due to infection with nontuberculous mycobacteria: a report of three cases. BMC Infect Dis 2020;20:295. https://doi.org/10.1186/s12879-020-05015-5. As previously stated, DUWL-sourced procedural water should not be used for surgical procedures, which expose normally sterile areas to the oral environment and present a greater risk for infection than other procedures.
Non-surgical extractions, endodontic procedures, and periodontal therapy
There has been considerable debate on whether sterile solutions should be used for certain non-surgical procedures. Recent position papers and a white paper (OSAP) provide guidance for specific non-surgical procedures:
- Consider using sterile irrigant for all dental extractions except exfoliating deciduous teeth.28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27.
- For all endodontic procedures, including pulpal therapy, consider using a sterile and/or antimicrobial solution as the irrigant.28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27.,33American Association of Endodontists. AAE Position Statement on Vital Pulp, 2021. https://www.aae.org/wp-content/uploads/2021/05/VitalPulpTherapyPositionStatement_v2.pdf. ,34Academy of Pediatric Dentistry. Pulp Therapy for Primary and Immature Permanent Teeth. https://www.aapd.org/research/oral-health-policies--recommendations/pulp-therapy-for-primary-and-immature-permanent-teeth/.
Regarding the suitability of procedural water for scaling and root planing, periodontal maintenance, or other gingival procedures, clinical judgement on the use of sterile irrigant is recommended based on how invasive the procedure is and the patient’s immunocompetence.28Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27. In addition, it has been suggested that DHCP consider the use of sterile water for surgical and non-surgical treatment in immunocompromised patients.35Porteous NB, Redding SW, Jorgensen JH. Isolation of non-tuberculosis mycobacteria in treated dental unit waterlines. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98(1):40-4. doi: 10.1016/j.tripleo.2004.02.006.
Other considerations
At the current time there are no Standards on DUWL for DHCP. ISO 16954:2015 (ANSI/ADA Standard 167) describes test methods for DUWL biofilm treatment but not for the clinical setting nor upper limits for bacterial contamination.36International Organization for Standardization - ISO 16954:2015 Dentistry — Test Methods for Dental Unit Waterline Biofilm Treatment, International Organization for Standards, Geneva, Switzerland, July 2015. https://www.iso.org/standard/58009.html. An ANSI/ADA Standard on DUWL is currently in development for dental unit manufacturers.
The CDC guidelines on infection prevention in the dental setting (2003) include guidance on DUWL and have been widely promulgated into State Dental Acts, making them compulsory. A recent white paper on DUWL published by OSAP (now ADS) provides more detailed information and updated guidance on DUWL, testing and maintenance. However, in a recent survey (2024) with more than 700 respondents (dentists, dental hygienists and dental assistants), gaps in knowledge were reported on how to monitor, maintain and treat DUWL.37Vinh R, Azzolin KA, Stream SE et al. Dental unit waterline infection control practice and knowledge gaps. J Am Dent Assoc 2024;155(6):515-25. https://doi.org/10.1016/j.adaj.2024.02.011. Numerous states now require that dental healthcare personnel complete infection control courses that specifically include DUWL. Several states have also adopted Rules related to DUWL: most recently, the dental waterline quality rule on DUWL testing and remediation (July 2025) in Georgia31Georgia Board of Dentistry. Georgia Board of Dentistry Adopts Rule on Dental Unit Water Quality, August 29, 2025. https://gbd.georgia.gov/press-releases/2025-08-29/georgia-board-dentistry-adopts-rule-dental-unit-water-quality. . It is the responsibility of DHCP to know what is applicable in their location, and to follow all State and federal requirements related to DUWL.
Conclusions
The substantial number and range of microorganisms found in DUWL, the evidence that contaminated DUWL present a risk for infection, and the global threat of multi-drug resistance are together cause for concern. Recent guidelines and updates help to inform these processes, and mandatory continuing education and staff training aid competence and compliance. It is recommended that dental offices should create a written Policy and Standard Operating Procedure on monitoring and maintaining DUWL, documentation, recordkeeping, troubleshooting and corrective actions, and staff training.
In summary, it is essential that DUWL are appropriately managed and used to safeguard the health of patients and DHCP.
References
- 1.Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morb Mortal Wkly Rep 2003;52:RR17. ww.cdc.gov/mmwr/PDF/rr/rr5217.pdf.
- 2.U.S. Environmental Protection Agency, National Primary Drinking Water Regulations, 1999 Available at: http://www.epa.gov/OGWDW/wot/appa.html
- 3.Barbeau J, Tanguay R, Faucher E et al. Multiparametric Analysis of Waterline Contamination in Dental Units. Amer Soc Microbiol 1996;62(11):3954-9.
- 4.Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. In: Molinari JA, Harte JA, eds. Cottone’s practical infection control in dentistry. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams; 2005:63-75.
- 5.Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol 2009;106(5):1424-37. doi: 10.1111/j.1365-2672.2008.04100.x.
- 6.O’Donnell MJ, Boyle MA, Russell RJ, Coleman DC. Management of dental unit waterline biofilms in the 21st century. Future Microbiol 2011;6(10):1209-26. doi: 10.2217/fmb.11.104.
- 7.Pankhurst CL, Scully C, Samaranayake L. Dental Unit Water Lines and their Disinfection and Management: A Review. Dent Update 2017;44(4):284-5, 289-92. doi: 10.12968/denu.2017.44.4.284.
- 8.Bayani M, Raisolvaezin K, Almasi-Hashiani A, Mirhoseini SH. Bacterial biofilm prevalence in dental unit waterlines: a systematic review and meta-analysis. BMC Oral Health 2023;23 (1):158.
- 9.Barbot V, Robert A, Rodier M-H, Imbert C. Update on infectious risks associated with dental unit waterlines. FEMS Immunol Med Microbiol 2012;65(2):196-204.
- 10.Tuvo B, Totaro M, Cristina ML et al. Prevention and control of legionella and Pseudomonas spp. colonization in dental units. Pathogens 2020;9(4):1-12.
- 11.Ditommaso S, Giacomuzzi M, Ricciardi E et al. Colonization by Pseudomonas aeruginosa of dental unit water lines and its relationship with other bacteria: suggestions for microbiological monitoring. J Water Health 2019;17(4):532-9.
- 12.Peralta G, Tobin-D’Angelo M, Parham A et al. Notes from the Field: Mycobacterium abscessus Infections Among Patients of a Pediatric Dentistry Practice--Georgia, 2015. Morb Mortal Wkly Rep 2016;65(13):355-6.
- 13.Singh J, O’Donnell K, Nieves DJ et al. Invasive Mycobacterium abscessus Outbreak at a Pediatric Dental Clinic. Open Forum Infect Dis 2021;8(6):165. https://doi.org/10.1093/ofid/ofab165.
- 14.Schönning C, Jernberg C, Klingenberg D et al. Legionellosis acquired through a dental unit: a case study. J Hosp Infect 2017;96(1):89-92. https://doi.org/10.1016/j.jhin.2017.01.009.
- 15.Ricci ML, Fontana S, Pinci F et al. Pneumonia associated with a dental unit waterline. The Lancet 2012;379(9816):684.
- 16.Martin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J 1987; 163:152-4.
- 17.Bassetti M, Vena A, Croxatto A et al. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018;7:212527. doi: 10.7573/dic.212527.
- 18.Allison JR, Dowson C, Jakubovics NS et al. Waterline Disinfectants Reduce Dental Bioaerosols: A Multitracer Validation. J Dent Res 2022;101(10):1198-204. doi: 10.1177/00220345221093522.
- 19.Poolkerd W, Swatasuk B, Saengpitak M et al. Metataxonomics study of dental bioaerosols affected by waterline disinfection. BMC Oral Health 2024;24(1):1575. doi: 10.1186/s12903-024-05304-4.
- 20.Dutil S, Veillette M, Mériaux A et al. Aerosolization of mycobacteria and legionellae during dental treatment: low exposure despite dental unit contamination. Environ Microbiol 2007;9(11):2836-43.
- 21.Zemouri C, Volgenant CMC, Buijs MJ et al. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol 2020;12(1):1762040.
- 22.Fotos PG, Westfall HN, Snyder IS et al. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J Dent Res 1985;64(12):1382–5.
- 23.Estrich CG, Gruninger SE, Lipman RD. Rates and predictors of exposure to Legionella pneumophila in the United States among dental practitioners: 2002 through 2012. J Am Dent Assoc 2017;148(3):164-71.
- 24.Szymanska J. Exposure to bacterial endotoxin during conservative dental treatment. Ann Agric Environ Med 2005;12(1):137-9.
- 25.Pankhurst CL, Coulter W, Philpott-Howard JN et al. Evaluation of the potential risk of occupational asthma in dentists exposed to contaminated dental unit waterlines. Prim Dent Care 2005;12(2):53-9.
- 26.U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines
- 27.Ji XY, Fei CN, Zhang Y et al. Three key factors influencing the bacterial contamination of dental unit waterlines: a 6-year survey from 2012 to 2017. Int Dent J 2019;69(3):192-9. doi: 10.1111/idj.12456.
- 28.Mills SE, Zawada J, Porteous N. (Ed.) Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. J Dent Infect Control Safety; 2018;1:1-27.
- 29.O’Donnell MJ, Tuttlebee CM, Falkiner FR, Coleman DC. Bacterial contamination of dental chair units in a modern dental hospital caused by leakage from suction system hoses containing extensive biofilm. J Hosp Infect 2005;59(4):348-60.
- 30.Lizzadro J, Mazzotta M, Girolamini L et al. Comparison between two types of dental unit waterlines: how evaluation of microbiological contamination can support risk containment. Int J Environ Res Public Health 2019;16(3):1-14.
- 31.Georgia Board of Dentistry. Georgia Board of Dentistry Adopts Rule on Dental Unit Water Quality, August 29, 2025. https://gbd.georgia.gov/press-releases/2025-08-29/georgia-board-dentistry-adopts-rule-dental-unit-water-quality.
- 32.Pérez-Alfonzo R, Poleo Brito LE, Vergara MS et al. Odontogenic cutaneous sinus tracts due to infection with nontuberculous mycobacteria: a report of three cases. BMC Infect Dis 2020;20:295. https://doi.org/10.1186/s12879-020-05015-5.
- 33.American Association of Endodontists. AAE Position Statement on Vital Pulp, 2021. https://www.aae.org/wp-content/uploads/2021/05/VitalPulpTherapyPositionStatement_v2.pdf.
- 34.Academy of Pediatric Dentistry. Pulp Therapy for Primary and Immature Permanent Teeth. https://www.aapd.org/research/oral-health-policies--recommendations/pulp-therapy-for-primary-and-immature-permanent-teeth/.
- 35.Porteous NB, Redding SW, Jorgensen JH. Isolation of non-tuberculosis mycobacteria in treated dental unit waterlines. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98(1):40-4. doi: 10.1016/j.tripleo.2004.02.006.
- 36.International Organization for Standardization - ISO 16954:2015 Dentistry — Test Methods for Dental Unit Waterline Biofilm Treatment, International Organization for Standards, Geneva, Switzerland, July 2015. https://www.iso.org/standard/58009.html.
- 37.Vinh R, Azzolin KA, Stream SE et al. Dental unit waterline infection control practice and knowledge gaps. J Am Dent Assoc 2024;155(6):515-25. https://doi.org/10.1016/j.adaj.2024.02.011.
Resources
Association for Dental Safety (formerly the Organization for Safety, Asepsis and Prevention (OSAP)). Dental Water Quality, Organization for Safety Asepsis and Prevention White Paper and Recommendations –2018. Mills SE, Zawada J, Porteous N. (Ed.) J Dental Infection Control and Safety; 2018; 1:1-27.
Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Morbidity and Mortality Weekly Report 2003;52:RR17. www.cdc.gov/mmwr/PDF/rr/rr5217.pdf.
Centers for Disease Control and Prevention - Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care – 2016. www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care.pdf
U.S. Food and Drug Administration – Dental Unit Waterlines. https://www.fda.gov/medical-devices/dental-devices/dental-unit-waterlines
Login to access