Non-invasive/non-restorative treatment of caries lesions
Dental caries is a multifactorial, endemic disease and a significant global oral health burden with an estimated 60% to 90% of schoolchildren and the majority of adults impacted.1World Health Organization. Global oral health status report. Towards universal health coverage for oral health by 2030. 2022. Available at: https://www.who.int/publications/i/item/9789240061484. In the United States, the estimated caries prevalence among adults 20 to 64 years-of-age is 90%.2National Institute of Health. National Institute of Dental and Craniofacial Research. Dental Caries (Tooth Decay) in Adults (Ages 20 to 64 Years). Available at: https://www.nidcr.nih.gov/research/data-statistics/dental-caries/adults#:~:text=92%25%20of%20adults%2020%20to%2064%20have%20had,incomes%20and%20less%20education%20have%20more%20untreated%20decay. For individuals 2 to 19 years-of-age an estimated caries prevalence of 45.8% has been found.3Centers for Disease Control and Prevention. Dental Caries in Primary Teeth. Available at: https://www.cdc.gov/oralhealth/publications/OHSR-2019-dental-caries-primary-teeth.html. With respect to untreated dental caries, an estimated prevalence of 10%, 16% and 21.3%, respectively, has been found for individuals aged 2–5 years, in the primary dentition for children aged 6–8 years, and among adults.3Centers for Disease Control and Prevention. Dental Caries in Primary Teeth. Available at: https://www.cdc.gov/oralhealth/publications/OHSR-2019-dental-caries-primary-teeth.html.,4Bashir NZ. Update on the prevalence of untreated caries in the US adult population, 2017-2020. J Am Dent Assoc 2022;153(4):300-8. doi: 10.1016/j.adaj.2021.09.004. These statistics highlight the ongoing need for effective caries management.
The caries process
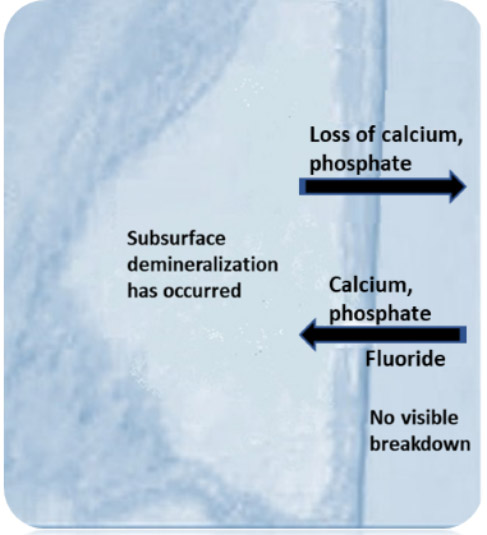
The caries process involves repeated cycles of demineralization and remineralization. Demineralization occurs due to exposure to acid produced through the bacterial metabolism of fermentable carbohydrates. Therefore, the presence of cariogenic bacteria and fermentable carbohydrates are prerequisites for dental caries.5Pitts NB, Zero DT, Marsh PD et al. Dental caries. Nat Rev Dis Primers 2017;25(3):17030. The initiation and progression of dental caries depends on the balance between risk factors and protective factors, and occurs when loss of calcium and phosphate from the dental hard tissue (demineralization) outpaces remineralization.6American Dental Association. Caries Risk Assessment Form (Age 0-6). Available at: https://www.ada.org/~/media/ADA/Member%20Center/FIles/topics_caries_under6.pdf.,7American Dental Association. Caries Risk Assessment Form (Age >6). Available at: http://www.ada.org/~/media/ADA/Science%20and%20Research/Files/topic_caries_over6.ashx. During the initial phase of lesion formation, the surface appears visibly intact while subsurface demineralization is present. Remineralization occurs when calcium and phosphate enter the area, if conditions are favorable. In the presence of fluoride this is accelerated, with fluoride adsorbing to the partially demineralized surface and attracting calcium ions, which in turn attract phosphate. These then enter the tooth, remineralizing it and the fluoride taken up results in the formation of fluorapatite. (Figure 1) In order to determine the destructive and protective factors involved and caries risk level for an individual patient, a periodic caries risk assessment should be performed.8AAPD. Caries-risk Assessment and Management for Infants, Children, and Adolescents. Latest revision, 2019. Available at: https://www.aapd.org/media/Policies_Guidelines/BP_CariesRiskAssessment.pdf
Non-cavitated caries lesions are also referred to as initial caries lesions/incipient lesions/enamel lesions. ‘Enamel lesion’ is, however, not synonymous with non-cavitated lesions since these are not all in/only in enamel.9Machiulskiene V, Campus G, Carvalho JC et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res 2020;54(1):7-14. doi: 10.1159/000503309. Non-cavitated caries lesions have incurred no loss of integrity of the tooth (visible breakdown).10Ferreira Zandona AG, Ritter AV, Eidson RS. Dental caries: Etiology, caries risk assessment, and management. Ch2, p43. In: Ritter AV, Boushell LW, Walter R. Sturdevant's art & science of operative dentistry-e-book. 7th Ed. Elsevier Health Sciences, 2017. Conversely, cavitated caries lesions are advanced lesions and demonstrate loss of integrity of the tooth, i.e., cavitation.10Ferreira Zandona AG, Ritter AV, Eidson RS. Dental caries: Etiology, caries risk assessment, and management. Ch2, p43. In: Ritter AV, Boushell LW, Walter R. Sturdevant's art & science of operative dentistry-e-book. 7th Ed. Elsevier Health Sciences, 2017. Under the International Caries Detection and Staging method (ICDAS) coronal caries lesions are categorized as initial, moderate or extensive based on visual and radiographic evaluation.11International Caries Classification and Management System. ICCMS™ Quick Reference Guide for Practitioners and Educators. https://iccms-web.com/uploads/asset/5928404ea4df6343406124.pdf. Initial lesions are ICDAS 1 or 2, equivalent to the ‘initial’ lesion described as visually non-cavitated under the American Dental Association Caries Classification System.12Young DA, Nový BB, Zeller GG et al. The American Dental Association Caries Classification System for Clinical Practice. A report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2015;146(2):79-86. http://dx.doi.org/10.1016/j.adaj.2014.11.018. ICDAS 3 and 4 are ‘moderate lesions’ and may or may not be cavitated. For approximal lesions, the radiolucency on radiographs extends to the dentinoenamel junction or outer third of dentin for ICDAS 1 and 2 and into the middle-third of the dentin for ICDAS 3 and 4.13CariesCare International. Caries staging and activity. Available at: https://cariescareinternational.com/2nd-d-detect-assess-caries-staging-and-activity1/. ICDAS 5 and 6 are extensive (advanced) caries lesions with visible cavitation.11International Caries Classification and Management System. ICCMS™ Quick Reference Guide for Practitioners and Educators. https://iccms-web.com/uploads/asset/5928404ea4df6343406124.pdf. More information on ICDAS staging, visual signs and assessment, and protocols can be found on the relevant websites.11International Caries Classification and Management System. ICCMS™ Quick Reference Guide for Practitioners and Educators. https://iccms-web.com/uploads/asset/5928404ea4df6343406124.pdf.,13CariesCare International. Caries staging and activity. Available at: https://cariescareinternational.com/2nd-d-detect-assess-caries-staging-and-activity1/.
Caries management
Caries prevention and control measures recommended for individual patients include oral hygiene instructions and thorough home care, as well as dietary and lifestyle advice. Other measures can include applications of in-office topical fluorides and home use of OTC/Rx gels, toothpastes, and rinses; resin infiltration; sealants; and other interventions.14Weyant RJ, Tracy SL, Anselmo TT et al; American Dental Association Council on Scientific Affairs Expert Panel on Topical Fluoride Caries Preventive Agents. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc 2013;144(11):1279-91. doi: 10.14219/jada.archive.2013.0057. Erratum in: J Am Dent Assoc 2013;144(12):1335. Dosage error in article text. Selection of management options is based on numerous factors and, among these, at the lesion level it is necessary to evaluate the site, stage/progression and activity.9Machiulskiene V, Campus G, Carvalho JC et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res 2020;54(1):7-14. doi: 10.1159/000503309.,12Young DA, Nový BB, Zeller GG et al. The American Dental Association Caries Classification System for Clinical Practice. A report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2015;146(2):79-86. http://dx.doi.org/10.1016/j.adaj.2014.11.018.,15Carvalho JC, Ekstrand KR, Thylstrup A. Dental plaque and caries on occlusal surfaces of first permanent molars in relation to stage of eruption. J Dent Res 1989;68(5):773-9. Guidelines on the non-restorative (noninvasive/microinvasive) management of non-cavitated and cavitated caries lesions have been published by the American Dental Association.16Slayton RL, Urquhart O, Araujo MWB et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837-49. e19. doi: 10.1016/j.adaj.2018.07.002. For the arrestment or reversal of non-cavitated lesions, the guidelines are surface-specific.
Non-cavitated coronal lesions
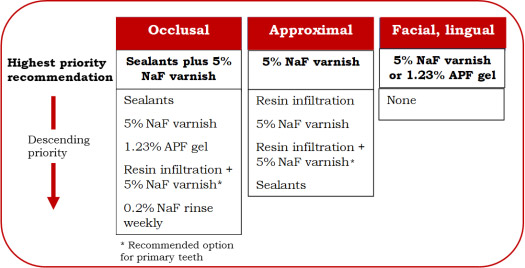
Occlusal – Application of sealants plus application every 3 to 6 months of 5% sodium fluoride (NaF) varnish is the highest priority recommendation. Other options in descending order of priority include sealants, 5% NaF varnish, and 1.23% APF gel. For the primary dentition, resin infiltration plus 5% NaF varnish is a further option. The lowest priority is weekly use of 0.2% NaF rinse.
Approximal – the highest priority recommendation is application of 5% NaF varnish. Lower priority options include resin infiltration, and sealants. For the primary dentition, resin infiltration plus 5% NaF varnish is a further option.
Facial and lingual – application of 5% NaF varnish or 1.23% APF gel. (Figure 2)
Cavitated coronal lesions
For cavitated coronal lesions in primary and permanent teeth, 6-monthly application of 38% silver diamine fluoride (SDF) is prioritized over 5% NaF varnish applications weekly for 3 weeks.
Non-cavitated and cavitated root caries lesions
The use of 5,000 ppm fluoride toothpaste/gel at least once daily is the highest priority recommendation for the arrestment and reversal of root caries. Other options, in descending order of priority, include 5% NaF varnish (every 3-6 months), a two-step annual application of 38% SDF followed by potassium iodide, 38% SDF annually, and 1% chlorhexidine+1% thymol varnish (every 3-6 months).
Basis for the ADA Guidelines
Recommendations in the ADA guidelines are ranked based on data on efficacy, resource efficiency, patient values and preferences, and feasibility.16Slayton RL, Urquhart O, Araujo MWB et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837-49. e19. doi: 10.1016/j.adaj.2018.07.002.,17Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014. A systematic review of 44 randomized controlled trials (RCT) conducted from 1984 to 2018, while published after publication of the guidelines, provided input for the guidelines.17Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014. (Table 1)
Non-cavitated caries lesions
Eight RCT were included on occlusal caries interventions, with 7 included in a network meta-analysis. Sealant application plus use of 5% NaF varnish provided a more than three-fold likelihood of caries arrestment/reversal (RR 3.35) compared to no treatment.17Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014. For other recommended interventions, the likelihood of arrestment/reversal ranged from two- to three-fold. For approximal lesions, to compare interventions, a network meta-analysis (NMA) was conducted on 6 of 13 related RCT in the review. Lesions were assessed as confined to the enamel or in the outer-third of the dentin. In comparing use of resin infiltration plus 5% NaF varnish to 5% NaF varnish alone, it was determined that the likelihood of lesion arrestment/reversal could be five-fold (1 study) and two-fold (2 studies), respectively. However, ‘certainty’ was graded as very low. Resin infiltration or sealant use resulted in a two-fold chance of reversal or arrestment, with low certainty. For facial and lingual lesions, a two- to three-fold likelihood of arrestment/reversal was found for use of 5% NaF varnish or 1.23% APF gel.
Cavitated coronal lesions
Four RCT were included in the systematic review. At 2.5 years, twice-yearly application of 38% SDF was found to be more effective than annual applications in arresting lesions. As noted in the review, the recommendation for cavitated coronal lesions in the permanent dentition was based on extrapolation of results for the primary dentition.
Root caries lesions
Seven of 11 RCT on various interventions were included in the meta-analysis. Based on 4 of the RCT, use of 5,000 ppm toothpaste or gel resulted in a three-fold likelihood of arresting/reversing non-cavitated and cavitated root caries lesions compared to no treatment. Results for other interventions across RCT yielded a two- to three-fold greater likelihood, however with very low certainty.17Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014.
| Table 1. Priority interventions evaluated in a systematic review17Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014. | |
|---|---|
| Non-cavitated occlusal lesions | Three-fold likelihood of caries arrestment/ reversal with sealant application plus use of 5% NaF varnish vs. no treatment. |
| Non-cavitated approximal lesions | Possibly five-fold likelihood of arrestment/reversal with resin infiltration plus 5% NaF varnish; possibly two-fold for 5% NaF varnish; both very low certainty. |
| Non-cavitated facial/ lingual lesions | Two- to three-fold likelihood of arrestment/reversal with use of 5% NaF varnish or 1.23% APF gel. |
| Cavitated coronal lesions | At 2.5 years, twice-yearly application of 38% SDF was found to be more effective than annual applications in arresting lesions in the primary dentition. |
| Root caries lesions | Three-fold likelihood of arresting/reversing non-cavitated and cavitated lesions with use of 5,000 ppm toothpaste or gel compared to no treatment. |
Additional reviews and studies
A more recent systematic review assessed the results of thirty-five RCT conducted between mid-2017 and March 2022.18Cabalén MB, Molina GF, Bono A, Burrow MF. Nonrestorative Caries Treatment: A Systematic Review Update. Int Dent J 2022;72(6):746-64. doi: 10.1016/j.identj.2022.06.022. Overall, SDF demonstrated greater efficacy than 5% NaF varnish in arresting dentinal caries, while 5% NaF varnish was an effective intervention for arresting lesions in enamel. The results also confirmed the efficacy of fissure sealants on occlusal surfaces, with similar efficacy found for resin-based and glass ionomer sealants. In addition, based on 10 RCT, resin infiltration was effective as an intervention for proximal lesions in dentin in both the primary and permanent detention. Caries arrestment rates ranged from 37.5% to 64.1% at 12 months and 48% at 30 months. For white-spot lesions, 5% NaF varnish and resin infiltration were both effective (no significant difference).18Cabalén MB, Molina GF, Bono A, Burrow MF. Nonrestorative Caries Treatment: A Systematic Review Update. Int Dent J 2022;72(6):746-64. doi: 10.1016/j.identj.2022.06.022.
In an umbrella review of systematic reviews conducted between 1970 and 2018,19Seifo N, Cassie H, Radford JR, Innes NPT. Silver diamine fluoride for managing carious lesions: an umbrella review. BMC Oral Health 2019;19(1):145. doi: 10.1186/s12903-019-0830-5. use of SDF was superior to fluoride varnish, ART, and placebo in arresting caries lesions in the primary dentition. Caries arrestment ranged from 65% to over 90% with use of SDF. Insufficient evidence was found to determine the effect of SDF on the permanent dentition. In a second umbrella review, twice-yearly application of SDF was found to be between 53% and 91% effective in primary teeth, depending on the included review.20BaniHani A, Santamaría RM, Hu S et al. Minimal intervention dentistry for managing carious lesions into dentine in primary teeth: an umbrella review. Eur Arch Paediatr Dent 2022;23(5):667-93. doi: 10.1007/s40368-021-00675-6. The American Academy of Pediatric Dentistry supports the use of SDF for caries arrestment.21American Academy of Pediatric Dentistry. Policy on the use of silver diamine fluoride for pediatric dental patients. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2022:72-5. Available at: https://www.aapd.org/media/Policies_Guidelines/P_SilverDiamine.pdf.
An ORCA/EFCD consensus statement (2020) on proximal caries in the permanent dentition was published, based on a systematic review and meta-analysis of reviews and RCT.22Splieth CH, Kanzow P, Wiegand A et al. How to intervene in the caries process: proximal caries in adolescents and adults-a systematic review and meta-analysis. Clin Oral Investig 2020;24(5):1623-36. doi: 10.1007/s00784-020-03201-y. It stated that fluoride and biofilm management reduced the likelihood of lesions progressing, and that proximal sealants or resin infiltration (which are both micro-invasive) were more effective than no treatment or non-invasive treatment. In a study with 193 adolescents and a follow-up of 4 to 5 years, almost half of more than 1100 permanent molars were identified with non-cavitated inactive occlusal lesions at baseline.23Zenkner JEA, Dalla Nora A, Alves LS et al. Long-term follow-up of inactive occlusal caries lesions: 4-5-year results. Clin Oral Invest 2019;23:847-53. https://doi.org/10.1007/s00784-018-2498-7. Most lesions did not progress. At the subject level, approximately 70% of children had only sound surfaces/inactive lesions at follow-up (no extractions, restorations, or active lesions).
Other considerations
As noted above, in addition to efficacy, the resource efficiency, patient values and preferences, and feasibility of an intervention are considered.16Slayton RL, Urquhart O, Araujo MWB et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837-49. e19. doi: 10.1016/j.adaj.2018.07.002.,17Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014. Non-invasive interventions are the least resource intensive and, along with micro-invasive interventions, are preferable given the preservation of tooth structure. Non-invasive/minimally invasive options are also more desirable and comfortable for patients.
There has been debate over whether sealants should be placed over early caries lesions. In doing so, the lesion is isolated from the oral cavity provided there is integrity of the sealant. In addition, any bacteria present would also be sealed, isolating them from the fermentable carbohydrates they need to produce acid. Furthermore, current evidence supports the efficacy of sealants on sound teeth and those with non-cavitated lesions. Sealant integrity needs to be assessed at recalls and further care provided if the sealant no longer functions (chipped or (partially) lost).
SDF is used in children and to treat root caries in adults. Caries removal is not required prior to application, and the application is relatively easy and painless.24Torres PJ, Phan HT, Bojorquez AK et al. Minimally Invasive Techniques Used for Caries Management in Dentistry. A Review. J Clin Pediatr Dent 2021;45(4):224-32. doi: 10.17796/1053-4625-45.4.2. This intervention is less traumatic for children than alternatives and helps with patient cooperation. Patient preferences help to inform the prioritization of a two-step protocol with annual application of 38% SDF followed by potassium iodide which results in minimal tooth stain, over a one-step SDF application that is associated with grey/dark grey tooth stain. The acceptability of the discoloration is higher for posterior than anterior teeth and additionally for parents when a child’s cooperation is lacking during treatment, information was provided ahead of time, and where the alternative was general anesthesia.25Sabbagh H, Othman M, Khogeer L et al. Parental acceptance of silver Diamine fluoride application on primary dentition: a systematic review and meta-analysis. BMC Oral Health 2020;20(1):227. doi: 10.1186/s12903-020-01195-3.,26Crystal YO, Janal MN, Hamilton DS, Niederman R. Parental perceptions and acceptance of silver diamine fluoride staining. J Am Dent Assoc 2017;148(7):510-8.e4. doi: 10.1016/j.adaj.2017.03.013. Patients/parents must be informed of staining associated with SDF and provide written informed consent before treatment is provided.27American Academy of Pediatric Dentistry. Chairside guide: Silver diamine fluoride in the management of dental caries lesions. Pediatr Dent 2018;40(6):492-3.
Conclusions
Non-restorative interventions for non-cavitated and cavitated lesions avoid/minimize loss of tooth structure and can remove a cycle of expanded restorative care from the equation. They are also supported by recent research. Further research using robust designs with clear diagnostic criteria and objective evaluations would further inform care. Recent research on diagnoses involving adjunctive use of AI has been promising.
It is essential that patients attend on an ongoing basis for evaluation and care, as well as for periodic caries risk assessment since risk level is dynamic. The likelihood of patient compliance with home care as well as return visits and recalls must also be considered when determining appropriate interventions for a given patient. It is recommended that non-restorative (non-invasive/ minimally invasive) interventions be provided when possible.28Schwendicke F, Walsh T, Lamont T et al. Interventions for treating cavitated or dentine carious lesions. Cochrane Database of Systematic Rev 2021;7. doi: 10.1002/14651858.CD013039.pub2.CD013039.,29Innes NPT, Frencken JE, Bjørndal L et al. Managing carious lesions: consensus recommendations on terminology. Adv Dent Res 2016;28(2):49-57. doi: 10.1177/0022034516639276. Consideration of individual patient factors, preferences and values are part of the clinical decision-making and collaborative process in agreeing on appropriate interventions.
References
- 1.World Health Organization. Global oral health status report. Towards universal health coverage for oral health by 2030. 2022. Available at: https://www.who.int/publications/i/item/9789240061484.
- 2.National Institute of Health. National Institute of Dental and Craniofacial Research. Dental Caries (Tooth Decay) in Adults (Ages 20 to 64 Years). Available at: https://www.nidcr.nih.gov/research/data-statistics/dental-caries/adults#:~:text=92%25%20of%20adults%2020%20to%2064%20have%20had,incomes%20and%20less%20education%20have%20more%20untreated%20decay.
- 3.Centers for Disease Control and Prevention. Dental Caries in Primary Teeth. Available at: https://www.cdc.gov/oralhealth/publications/OHSR-2019-dental-caries-primary-teeth.html.
- 4.Bashir NZ. Update on the prevalence of untreated caries in the US adult population, 2017-2020. J Am Dent Assoc 2022;153(4):300-8. doi: 10.1016/j.adaj.2021.09.004.
- 5.Pitts NB, Zero DT, Marsh PD et al. Dental caries. Nat Rev Dis Primers 2017;25(3):17030.
- 6.American Dental Association. Caries Risk Assessment Form (Age 0-6). Available at: https://www.ada.org/~/media/ADA/Member%20Center/FIles/topics_caries_under6.pdf.
- 7.American Dental Association. Caries Risk Assessment Form (Age >6). Available at: http://www.ada.org/~/media/ADA/Science%20and%20Research/Files/topic_caries_over6.ashx.
- 8.AAPD. Caries-risk Assessment and Management for Infants, Children, and Adolescents. Latest revision, 2019. Available at: https://www.aapd.org/media/Policies_Guidelines/BP_CariesRiskAssessment.pdf
- 9.Machiulskiene V, Campus G, Carvalho JC et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res 2020;54(1):7-14. doi: 10.1159/000503309.
- 10.Ferreira Zandona AG, Ritter AV, Eidson RS. Dental caries: Etiology, caries risk assessment, and management. Ch2, p43. In: Ritter AV, Boushell LW, Walter R. Sturdevant's art & science of operative dentistry-e-book. 7th Ed. Elsevier Health Sciences, 2017.
- 11.International Caries Classification and Management System. ICCMS™ Quick Reference Guide for Practitioners and Educators. https://iccms-web.com/uploads/asset/5928404ea4df6343406124.pdf.
- 12.Young DA, Nový BB, Zeller GG et al. The American Dental Association Caries Classification System for Clinical Practice. A report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2015;146(2):79-86. http://dx.doi.org/10.1016/j.adaj.2014.11.018.
- 13.CariesCare International. Caries staging and activity. Available at: https://cariescareinternational.com/2nd-d-detect-assess-caries-staging-and-activity1/.
- 14.Weyant RJ, Tracy SL, Anselmo TT et al; American Dental Association Council on Scientific Affairs Expert Panel on Topical Fluoride Caries Preventive Agents. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc 2013;144(11):1279-91. doi: 10.14219/jada.archive.2013.0057. Erratum in: J Am Dent Assoc 2013;144(12):1335. Dosage error in article text.
- 15.Carvalho JC, Ekstrand KR, Thylstrup A. Dental plaque and caries on occlusal surfaces of first permanent molars in relation to stage of eruption. J Dent Res 1989;68(5):773-9.
- 16.Slayton RL, Urquhart O, Araujo MWB et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837-49. e19. doi: 10.1016/j.adaj.2018.07.002.
- 17.Urquhart O, Tampi MP, Pilcher L et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2019;98(1):14-26. doi: 10.1177/0022034518800014.
- 18.Cabalén MB, Molina GF, Bono A, Burrow MF. Nonrestorative Caries Treatment: A Systematic Review Update. Int Dent J 2022;72(6):746-64. doi: 10.1016/j.identj.2022.06.022.
- 19.Seifo N, Cassie H, Radford JR, Innes NPT. Silver diamine fluoride for managing carious lesions: an umbrella review. BMC Oral Health 2019;19(1):145. doi: 10.1186/s12903-019-0830-5.
- 20.BaniHani A, Santamaría RM, Hu S et al. Minimal intervention dentistry for managing carious lesions into dentine in primary teeth: an umbrella review. Eur Arch Paediatr Dent 2022;23(5):667-93. doi: 10.1007/s40368-021-00675-6.
- 21.American Academy of Pediatric Dentistry. Policy on the use of silver diamine fluoride for pediatric dental patients. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2022:72-5. Available at: https://www.aapd.org/media/Policies_Guidelines/P_SilverDiamine.pdf.
- 22.Splieth CH, Kanzow P, Wiegand A et al. How to intervene in the caries process: proximal caries in adolescents and adults-a systematic review and meta-analysis. Clin Oral Investig 2020;24(5):1623-36. doi: 10.1007/s00784-020-03201-y.
- 23.Zenkner JEA, Dalla Nora A, Alves LS et al. Long-term follow-up of inactive occlusal caries lesions: 4-5-year results. Clin Oral Invest 2019;23:847-53. https://doi.org/10.1007/s00784-018-2498-7.
- 24.Torres PJ, Phan HT, Bojorquez AK et al. Minimally Invasive Techniques Used for Caries Management in Dentistry. A Review. J Clin Pediatr Dent 2021;45(4):224-32. doi: 10.17796/1053-4625-45.4.2.
- 25.Sabbagh H, Othman M, Khogeer L et al. Parental acceptance of silver Diamine fluoride application on primary dentition: a systematic review and meta-analysis. BMC Oral Health 2020;20(1):227. doi: 10.1186/s12903-020-01195-3.
- 26.Crystal YO, Janal MN, Hamilton DS, Niederman R. Parental perceptions and acceptance of silver diamine fluoride staining. J Am Dent Assoc 2017;148(7):510-8.e4. doi: 10.1016/j.adaj.2017.03.013.
- 27.American Academy of Pediatric Dentistry. Chairside guide: Silver diamine fluoride in the management of dental caries lesions. Pediatr Dent 2018;40(6):492-3.
- 28.Schwendicke F, Walsh T, Lamont T et al. Interventions for treating cavitated or dentine carious lesions. Cochrane Database of Systematic Rev 2021;7. doi: 10.1002/14651858.CD013039.pub2.CD013039.
- 29.Innes NPT, Frencken JE, Bjørndal L et al. Managing carious lesions: consensus recommendations on terminology. Adv Dent Res 2016;28(2):49-57. doi: 10.1177/0022034516639276.