Pre-procedural Rinsing to Reduce Aerosolized Microbial Loads
Mouthrinses are available with preventive, therapeutic and cosmetic indications. Examples of chemotherapeutic rinses include fluoride rinses and anti-plaque anti-gingivitis rinses, available in over-the-counter and prescription formulations. Other rinses carry cosmetic claims such as anti-halitosis, whitening or cleansing. Rinses have also been used pre-procedurally. This is an off-label use, not approved by the U.S. Food and Drug Administration. To varying degrees, rinses are used pre-procedurally to reduce the intra-oral microbial load, to help prevent post-operative infections and to reduce the bacterial load in dental aerosols.1Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc 2019;150(12):1015-26.e1. doi: 10.1016/j.adaj.2019.06.024.,2Sreenivasan PK, Gittins E. The effects of a chlorhexidine mouthrinse on culturable micro-organisms of the tongue and saliva. Microbiol Res 2004;159(4):365-70.,3Larsen PE. The effect of a chlorhexidine rinse on the incidence of alveolar osteitis following the surgical removal of impacted mandibular third molars. J Oral Maxillofac Surg 1991;49(9):932-37. In a 2004 survey of dental professionals working in army dental clinics and using pre-procedural rinses, 38%, 21% and approximately 9% of respondents, respectively, perceived that the greatest benefits of pre-procedural rinsing were decreases in oral bacterial loads, the incidence of postoperative infections and bacterial aerosolization.4Hennessy B, Joyce A. A survey of preprocedural antiseptic mouth rinse use in Army dental clinics. Mil Med 2004;169(8):600-3. Standard and transmission-based precautions, isolation of the clinical site (where possible) and the use of high-volume evacuation reduce exposure to treatment-generated aerosols. Most recently, pre-procedural rinsing has also been recommended for use during the COVID-19 pandemic with the objective of reducing viral loads.5American Dental Association. Interim Guidance for Minimizing Risk of COVID-19 Transmission. Available at: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf.,6Meng L, Fang H, Bian Z.. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J Dent Res 2020;99. 002203452091424. 10.1177/0022034520914246.,7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9.
Aerosol Production in Dentistry
Aerosol production in the dental operatory is associated primarily with the use of ultrasonic scalers and high-speed handpieces.8Kobza J, Pastuszka JS, Brągoszewska E. Do exposures to aerosols pose a risk to dental professionals? Occupat Med 2018;68(7):454-8. The use of 3-in-1 air-water syringes and other devices also produces aerosols, as well as when individuals cough, sneeze, or gag. Aerosols are a source of pathogens for a number of diseases, such as tuberculosis, measles and influenza.9Tellier, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019;19:101. Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3707-y. Aerosols contain particles that are 50 microns or less in size.9Tellier, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019;19:101. Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3707-y. In addition, particles <10 microns are considered ‘inspirable’ and the most likely to reach the lower respiratory tract.9Tellier, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019;19:101. Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3707-y. The vast majority in dental aerosols are <5 microns, letting them travel significant distances from the treatment site.10Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol 1995;61(8):3165-8. Dental aerosols contain intraorally-derived microorganisms, which includes bacteria, viruses and fungi.
In one study in a multi-chair operatory testing air samples, a more than five-fold increase in the level of aerosolized bacteria was found during treatment sessions before decreasing post-treatment.11Al Maghlouth A, Al Yousef Y, Al Bagieh N. Qualitative and quantitative analysis of bacterial aerosols. J Contemp Dent Pract. 2004 Nov 15;5(4):91-100. In another study, bacterial aerosols reached up to more than 30 feet from a treatment site after initiation of treatment, and bacterial levels were still greater 3 hours after cessation of treatment than prior to treatment.10Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol 1995;61(8):3165-8. The emergence of SARS, MERS, and in particular the recent COVID-19 pandemic, has increased the focus and concern related to treatment-generated aerosols.5American Dental Association. Interim Guidance for Minimizing Risk of COVID-19 Transmission. Available at: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf.,6Meng L, Fang H, Bian Z.. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J Dent Res 2020;99. 002203452091424. 10.1177/0022034520914246.,7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9.
Pre-procedural Rinsing to Reduce the Bacterial Load in Aerosols
Mouthrinses used pre-procedurally to reduce bacterial loads in dental treatment-generated aerosols include chlorhexidine gluconate (CHX), cetylpyridinum chloride (CPC; quaternary ammonium compound), essential oils (EO) and povidone-iodine (PVP-I).1Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc 2019;150(12):1015-26.e1. doi: 10.1016/j.adaj.2019.06.024.,12Feres M, Figueiredo LC, Faveri M, Stewart B, de Vizio W. The effectiveness of a preprocedural mouthrinse containing cetylpyridinium chloride in reducing bacteria in the dental office. J Am Dent Assoc 2010;141:415-22.,13Domingo MA, Farrales MS, Loya RM, Pura MA, Uy H. The effect of 1% povidone-iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene. J Philipp Dent Assoc 1996;48(2):31-8. CHX has broad-spectrum antimicrobial efficacy. At bactericidal concentrations it disrupts bacterial cell walls, resulting in leakage of intracellular proteins and cell death. In addition, CHX offers substantivity for up to 12 hours by binding to oral hard and soft tissues, and salivary proteins.14DePaola LG, Eshenaur Spolarich A. Safety and Efficacy of Antimicrobial Mouthrinses in Clinical Practice. J Dent Hyg 2007;81(suppl 1) 117. Available at: https://jdh.adha.org/content/jdenthyg/81/suppl_1/117.full.pdf. CPC also exerts broad-spectrum activity, with disruption of the cell membrane, intracellular leakage, and cell death.15Scheie AA. Modes of action of currently known chemical antiplaque agents other than chlorhexidine. J Dent Res 1989;68 (Spec Iss):1609-16. It binds intraorally to a lesser extent than CHX.14DePaola LG, Eshenaur Spolarich A. Safety and Efficacy of Antimicrobial Mouthrinses in Clinical Practice. J Dent Hyg 2007;81(suppl 1) 117. Available at: https://jdh.adha.org/content/jdenthyg/81/suppl_1/117.full.pdf. The mechanisms of action of EO vary depending on the specific EO.16Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 2013;6(12):1451‐74. doi:10.3390/ph6121451. In general, EO have been found to work similarly, with disruption of cell membranes and damage membrane proteins, increase membrane permeability and cause leakage of intracellular components, leading to cell death.16Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 2013;6(12):1451‐74. doi:10.3390/ph6121451. In the case of PVP-I, the free iodine released penetrates cell membranes and interacts with proteins and fatty acids, resulting in cell death.17Prescribers Digital Reference. povidone iodine – Drug Summary. Available at: https://www.pdr.net/drug-summary/Betadine-5–povidone-iodine-2152.
Figure 1. Reductions in CFU/ml with pre-procedural rinsing
In a recent systematic review of 13 randomized controlled clinical trials, 12 of which were included in a meta-analysis, the efficacy of pre-procedural rinsing in reducing the bacterial load in dental treatment-generated aerosols was investigated.1Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc 2019;150(12):1015-26.e1. doi: 10.1016/j.adaj.2019.06.024. Compared to the control (water, placebo or no rinse), the number of colony-forming units per milliliter (CFU/ml) was reduced by a mean of 78.9%, 61.3% and 61.2%, respectively, for rinses containing CHX, EO and CPC. (Figure 1) While it was recognized that none of the studies were at low risk of bias, it was concluded that there was moderate evidence of their efficacy in reducing the bacterial load in treatment-generated aerosols.1Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc 2019;150(12):1015-26.e1. doi: 10.1016/j.adaj.2019.06.024.
Results of individual studies with pre-procedural use of these rinses for reductions in bacterial load in aerosols demonstrate their efficacy. In one study, a quaternary ammonium compound mouthrinse was found to result in an 89.3% reduction in aerosolized bacteria during treatment after a pre-procedural and procedural rinse compared to rinsing with sterile water.18Litsky BY, Mascis JD, Litsky W. Use of an antimicrobial mouthwash to minimize the bacterial aerosol contamination generated by the high-speed drill. PlumX Metrics. Available at: https://doi.org/10.1016/0030-4220(70)90407-X. In a subsequent study, rinsing with a quaternary ammonium compound, a phenol, EO, or zinc chloride was found to be effective in reducing aerosolized bacteria.19Wyler D, Miller RL, Micik RE. Efficacy of self-administered preoperative oral hygiene procedures in reducing the concentration of bacteria in aerosols generated during dental procedures. J Dent Res 1971;50(2):509. The mean reduction in bacteria was 97%. CHX was found in other studies to be effective in significantly reducing the level of aerosolized bacteria during dental procedures, and in one of these to be more effective than an EO mouthrinse.20Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc 1995;126(12):1634-9.,21Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria-containing spray produced during ultrasonic scaling. Gen Dent 2001;49(6):648-52.
Significant reductions in bacterial load in aerosols generated by ultrasonic scaling are found following pre-procedural rinsing with an EO mouthrinse.22Fine DH, Mendieta C, Barnett ML, Furgang D, Meyers R. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental aerosols. J Periodontol 1992;63(10):821-4. In one study, half-mouth ultrasonic scaling was performed for 10 minutes. Bacterial aerosolization without any pre-rinse (control) was compared with the same procedure conducted after pre-rinsing by measuring CFUs of bacteria recovered from the aerosols. Reductions in bacterial aerosolization of 94.1% and 33.9%, respectively, were found after pre-rinsing with EO or water. In the second study, compared to baseline (prior to rinsing), a reduction in bacterial CFU/ml of 92.1% and 91.3%, respectively, was found after pre-procedural rinsing at 5 minutes and 40 minutes.23Fine DH, Furgang D, Korik I, Olshan A, Barnett ML, Vincent JW. Reduction of viable bacteria in dental aerosols by preprocedural rinsing with an antiseptic mouthrinse. Am J Dent 1993;6(5):219-21. Other studies measuring salivary bacterial levels also lend support to the efficacy of pre-rinsing with antimicrobial rinses.24Altonen M, Saxen L, Kosunen T, Ainamo J. Effect of two antimicrobial rinses and oral prophylaxis on preoperative degerming of saliva. Int J Oral Surg 1976;5(6):276-84.,25Veksler AE, Kayrouz GA, Newman MG. Reduction of salivary bacteria by pre-procedural rinses with chlorhexidine 0.12%. J Periodontol 1991;62(11):649-51.,26Balbuena L, Stambaugh KI, Ramirez SG, Yeager C. Effects of topical oral antiseptic rinses on bacterial counts of saliva in healthy human subjects. Otolaryngol Head Neck Surg 1998;118(5):625-9.
Viral Aerosols and COVID-19
The causative agent for the COVID-19 pandemic, SARS-CoV-2, is transmitted primarily through droplets and contact.5American Dental Association. Interim Guidance for Minimizing Risk of COVID-19 Transmission. Available at: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf.,6Meng L, Fang H, Bian Z.. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J Dent Res 2020;99. 002203452091424. 10.1177/0022034520914246. SARS-CoV-2 is an enveloped (lipid layer) virus with single-stranded RNA. It has 4 proteins, one of which is the spike protein that fuses with receptors in human cells. The lipid membrane is integral to the functioning of the viral cell. High viral loads of SARS-CoV-2 have been found in saliva in the early phase of SARS-CoV-2 infection and in infected individuals during hospitalization.27Kirk-Bailey J, Combes J, Sunkaraneni S, Challacombe S. The use of Povidone Iodine nasal spray and mouthwash during the current COVID-19 pandemic for the reduction of cross infection and protection of healthcare workers. Last revised 24 April 2020. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092.,28To KK-W, Tsang OT-Y, Yip C-YC, Chan K-H, Wu T-C, Chan JM-C, et al. Consistent detection of 2019 novel coronavirus in saliva. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020;361:1319. doi:10.1093/cid/ciaa149. In one study, high viral loads were detected in the oropharynx of asymptomatic individuals.29Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med 2020;382:1177-9. Aerosols represent one of the other modes of transmission for SARS-CoV-2, and viable SARS-CoV-2 has been found in an aerosol up to 3 hours after treatment.30van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. Available at: https://www.nejm.org/doi/full/10.1056/NEJMc2004973. In a newly-published study on a hospital setting where COVID-19 patients were hospitalized, it was found that SARS-CoV-2 could travel several meters.31Guo Z-D, Wang Z-Y, Zhang S-F, Li X, Li L, Li C, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis 2020 Jul [date cited]. https://doi.org/10.3201/eid2607.200885. The test used did not measure the level of viable virus.
The use of pre-procedural rinsing with either hydrogen peroxide or PVP-I has been advocated, with the objective of reducing the viral load.5American Dental Association. Interim Guidance for Minimizing Risk of COVID-19 Transmission. Available at: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf.,7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9.,32Caruso AA, Del Prete A, Lazzarino AI, Capaldi R, Grumetto L. May hydrogen peroxide reduce the hospitalization rate and complications of SARS-CoV-2 infection? Letter to the Editor. Infection Control & Hospital Epidemiology as part of the Cambridge Coronavirus Collection. doi: 10.1017/ice.2020.170. Recommendations have included pre-procedural rinsing with 1.5% hydrogen peroxide or 0.2% PVP-I for patients visiting dental offices. In other geographic areas, 1% hydrogen peroxide has been recommended.7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9. The use of 3% hydrogen peroxide rinses has also been suggested in patients with early COVID-19, to reduce the viral load.32Caruso AA, Del Prete A, Lazzarino AI, Capaldi R, Grumetto L. May hydrogen peroxide reduce the hospitalization rate and complications of SARS-CoV-2 infection? Letter to the Editor. Infection Control & Hospital Epidemiology as part of the Cambridge Coronavirus Collection. doi: 10.1017/ice.2020.170. Hydrogen peroxide rinses have a long history of use with demonstrated safety.
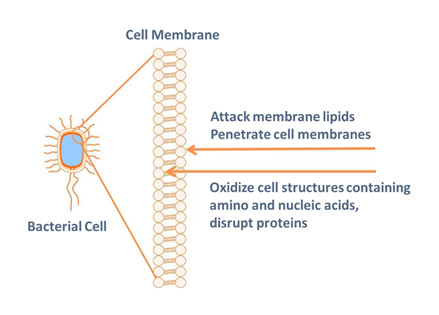
SARS-CoV-2 is susceptible to oxidation. This is the proposed mechanism of action for pre-procedural rinsing with hydrogen peroxide.7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9. Hydroxyl free radicals are produced by hydrogen peroxide and are known to attack membrane lipids, genetic material (which contains nucleic acid), and other cell components.33Centers for Disease Control and Prevention. Chemical Disinfectants
Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Available at:
https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html#Hydrogen.
PVP-I has demonstrated in vitro efficacy against SARS-CoV and MERS-CoV.27Kirk-Bailey J, Combes J, Sunkaraneni S, Challacombe S. The use of Povidone Iodine nasal spray and mouthwash during the current COVID-19 pandemic for the reduction of cross infection and protection of healthcare workers. Last revised 24 April 2020. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092. It was recommended against MERS-CoV during the MERS epidemic.34Eggers, M. Infectious Disease Management and Control with Povidone Iodine. Infect Dis Ther 2019;8:581-93. PVP-I releases free (unbound) iodine. This oxidizes cell components containing lipids, and amino and nucleic acids which disrupts proteins.27Kirk-Bailey J, Combes J, Sunkaraneni S, Challacombe S. The use of Povidone Iodine nasal spray and mouthwash during the current COVID-19 pandemic for the reduction of cross infection and protection of healthcare workers. Last revised 24 April 2020. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092.,34Eggers, M. Infectious Disease Management and Control with Povidone Iodine. Infect Dis Ther 2019;8:581-93.,35Kanagalingam J, Feliciano R, Hah JH, Labib H, Le TA, Lin JC. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int J Clin Pract 2015;69(11):1247-56. doi: 10.1111/ijcp.12707.
Summary
Pre-procedural rinsing to reduce aerosolized bacteria has been recommended to help reduce the risk of aerosol-mediated transmission of pathogenic organisms from patients to dental professionals.1Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc 2019;150(12):1015-26.e1. doi: 10.1016/j.adaj.2019.06.024.,7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9.,19Wyler D, Miller RL, Micik RE. Efficacy of self-administered preoperative oral hygiene procedures in reducing the concentration of bacteria in aerosols generated during dental procedures. J Dent Res 1971;50(2):509. At the current time, there is no evidence for the use of any pre-procedural rinse against SARS-COV-2.5American Dental Association. Interim Guidance for Minimizing Risk of COVID-19 Transmission. Available at: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf.,7Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:9.,27Kirk-Bailey J, Combes J, Sunkaraneni S, Challacombe S. The use of Povidone Iodine nasal spray and mouthwash during the current COVID-19 pandemic for the reduction of cross infection and protection of healthcare workers. Last revised 24 April 2020. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092. Biologic plausibility, however, suggests that hydrogen peroxide and PVP-I could be expected to be virucidal intra-orally and may reduce SARS-CoV-2 viral load in dental aerosols. More research is required on SARS-CoV-2 and COVID-19 across a wide array of areas, including the in vivo efficacy of pre-procedural rinses. In addition, the quantifiable risk from aerosols and the minimal infectious dose for SARS-CoV-2 are as yet unknown. In the meantime, the avoidance, reduction, and mitigation of dental aerosols, as well as the reduction of aerosolized bacteria, viruses and fungi, is highly desirable.
References
- 1.Dominy SS, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333 https://www.ncbi.nlm.nih.gov/pubmed/30746447.
- 2.Sadrameli M, et al. Linking mechanisms of periodontitis to Alzheimer’s disease. Curr Opin Neurol. 2020;33(2):230-8 https://www.ncbi.nlm.nih.gov/pubmed/32097126.
- 3.Borsa L, et al. Analysis the link between periodontal diseases and Alzheimer’s disease: A systematic review. Int J Environ Res Public Health. 2021;18(17) https://www.ncbi.nlm.nih.gov/pubmed/34501899.
- 4.Costa MJF, et al. Relationship of Porphyromonas gingivalis and Alzheimer’s disease: A systematic review of pre-clinical studies. Clin Oral Investig. 2021;25(3):797-806 https://www.ncbi.nlm.nih.gov/pubmed/33469718.
- 5.Munoz Fernandez SS, Lima Ribeiro SM. Nutrition and Alzheimer disease. Clin Geriatr Med. 2018;34(4):677-97 https://www.ncbi.nlm.nih.gov/pubmed/30336995.
- 6.Aquilani R, et al. Is the Brain Undernourished in Alzheimer’s Disease? Nutrients. 2022;14(9) https://www.ncbi.nlm.nih.gov/pubmed/35565839.
- 7.Fukushima-Nakayama Y, et al. Reduced mastication impairs memory function. J Dent Res. 2017;96(9):1058-66 https://www.ncbi.nlm.nih.gov/pubmed/28621563.
- 8.Kim HB, et al. Abeta accumulation in vmo contributes to masticatory dysfunction in 5XFAD Mice. J Dent Res. 2021;100(9):960-7 https://www.ncbi.nlm.nih.gov/pubmed/33719684.
- 9.Miura H, et al. Relationship between cognitive function and mastication in elderly females. J Oral Rehabil. 2003;30(8):808-11 https://www.ncbi.nlm.nih.gov/pubmed/12880404.
- 10.Lexomboon D, et al. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60(10):1951-6 https://www.ncbi.nlm.nih.gov/pubmed/23035667.
- 11.Elsig F, et al. Tooth loss, chewing efficiency and cognitive impairment in geriatric patients. Gerodontology. 2015;32(2):149-56 https://www.ncbi.nlm.nih.gov/pubmed/24128078.
- 12.Kim EK, et al. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch Gerontol Geriatr. 2017;70:209-13 https://www.ncbi.nlm.nih.gov/pubmed/28214402.
- 13.Kim MS, et al. The association between mastication and mild cognitive impairment in Korean adults. Medicine (Baltimore). 2020;99(23):e20653 https://www.ncbi.nlm.nih.gov/pubmed/32502052.
- 14.Cardoso MG, et al. Relationship between functional masticatory units and cognitive impairment in elderly persons. J Oral Rehabil. 2019;46(5):417-23 https://www.ncbi.nlm.nih.gov/pubmed/30614023.
- 15.Popovac A, et al. Oral health status and nutritional habits as predictors for developing alzheimer’s disease. Med Princ Pract. 2021;30(5):448-54 https://www.ncbi.nlm.nih.gov/pubmed/34348313.
- 16.Park T, et al. More teeth and posterior balanced occlusion are a key determinant for cognitive function in the elderly. Int J Environ Res Public Health. 2021;18(4) https://www.ncbi.nlm.nih.gov/pubmed/33669490.
- 17.Lin CS, et al. Association between tooth loss and gray matter volume in cognitive impairment. Brain Imaging Behav. 2020;14(2):396-407 https://www.ncbi.nlm.nih.gov/pubmed/32170642.
- 18.Kumar S, et al. Oral health status and treatment need in geriatric patients with different degrees of cognitive impairment and dementia: a cross-sectional study. J Family Med Prim Care. 2021;10(6):2171-6 https://www.ncbi.nlm.nih.gov/pubmed/34322409.
- 19.Delwel S, et al. Chewing efficiency, global cognitive functioning, and dentition: A cross-sectional observational study in older people with mild cognitive impairment or mild to moderate dementia. Front Aging Neurosci. 2020;12:225 https://www.ncbi.nlm.nih.gov/pubmed/33033478.
- 20.Da Silva JD, et al. Association between cognitive health and masticatory conditions: a descriptive study of the national database of the universal healthcare system in Japan. Aging (Albany NY). 2021;13(6):7943-52 https://www.ncbi.nlm.nih.gov/pubmed/33739304.
- 21.Galindo-Moreno P, et al. The impact of tooth loss on cognitive function. Clin Oral Investig. 2022;26(4):3493-500 https://www.ncbi.nlm.nih.gov/pubmed/34881401.
- 22.Stewart R, et al. Adverse oral health and cognitive decline: The health, aging and body composition study. J Am Geriatr Soc. 2013;61(2):177-84 https://www.ncbi.nlm.nih.gov/pubmed/23405916.
- 23.Dintica CS, et al. The relation of poor mastication with cognition and dementia risk: A population-based longitudinal study. Aging (Albany NY). 2020;12(9):8536-48 https://www.ncbi.nlm.nih.gov/pubmed/32353829.
- 24.Kim MS, Han DH. Does reduced chewing ability efficiency influence cognitive function? Results of a 10-year national cohort study. Medicine (Baltimore). 2022;101(25):e29270 https://www.ncbi.nlm.nih.gov/pubmed/35758356.
- 25.Ko KA, et al. The Impact of Masticatory Function on Cognitive Impairment in Older Patients: A Population-Based Matched Case-Control Study. Yonsei Med J. 2022;63(8):783-9 https://www.ncbi.nlm.nih.gov/pubmed/35914761.
- 26.Garre-Olmo J. [Epidemiology of Alzheimer’s disease and other dementias]. Rev Neurol. 2018;66(11):377-86 https://www.ncbi.nlm.nih.gov/pubmed/29790571.
- 27.Stephan BCM, et al. Secular Trends in Dementia Prevalence and Incidence Worldwide: A Systematic Review. J Alzheimers Dis. 2018;66(2):653-80 https://www.ncbi.nlm.nih.gov/pubmed/30347617.
- 28.Lopez OL, Kuller LH. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer’s disease and other dementias. Handb Clin Neurol. 2019;167:139-48 https://www.ncbi.nlm.nih.gov/pubmed/31753130.
- 29.Ono Y, et al. Occlusion and brain function: mastication as a prevention of cognitive dysfunction. J Oral Rehabil. 2010;37(8):624-40 https://www.ncbi.nlm.nih.gov/pubmed/20236235.
- 30.Kubo KY, et al. Masticatory function and cognitive function. Okajimas Folia Anat Jpn. 2010;87(3):135-40 https://www.ncbi.nlm.nih.gov/pubmed/21174943.
- 31.Chen H, et al. Chewing Maintains Hippocampus-Dependent Cognitive Function. Int J Med Sci. 2015;12(6):502-9 https://www.ncbi.nlm.nih.gov/pubmed/26078711.
- 32.Azuma K, et al. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int J Mol Sci. 2017;18(8) https://www.ncbi.nlm.nih.gov/pubmed/28771175.
- 33.Chuhuaicura P, et al. Mastication as a protective factor of the cognitive decline in adults: A qualitative systematic review. Int Dent J. 2019;69(5):334-40 https://www.ncbi.nlm.nih.gov/pubmed/31140598.
- 34.Lopez-Chaichio L, et al. Oral health and healthy chewing for healthy cognitive ageing: A comprehensive narrative review. Gerodontology. 2021;38(2):126-35 https://www.ncbi.nlm.nih.gov/pubmed/33179281.
- 35.Tada A, Miura H. Association between mastication and cognitive status: A systematic review. Arch Gerontol Geriatr. 2017;70:44-53 https://www.ncbi.nlm.nih.gov/pubmed/28042986.
- 36.Ahmed SE, et al. Influence of Dental Prostheses on Cognitive Functioning in Elderly Population: A Systematic Review. J Pharm Bioallied Sci. 2021;13(Suppl 1):S788-S94 https://www.ncbi.nlm.nih.gov/pubmed/34447202.
- 37.Tonsekar PP, et al. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology. 2017;34(2):151-63 https://www.ncbi.nlm.nih.gov/pubmed/28168759.
- 38.Nangle MR, Manchery N. Can chronic oral inflammation and masticatory dysfunction contribute to cognitive impairment? Curr Opin Psychiatry. 2020;33(2):156-62 https://www.ncbi.nlm.nih.gov/pubmed/31895157.
- 39.Nakamura T, et al. Oral dysfunctions and cognitive impairment/dementia. J Neurosci Res. 2021;99(2):518-28 https://www.ncbi.nlm.nih.gov/pubmed/33164225.
- 40.Weijenberg RAF, et al. Mind your teeth-The relationship between mastication and cognition. Gerodontology. 2019;36(1):2-7 https://www.ncbi.nlm.nih.gov/pubmed/30480331.
- 41.Asher S, et al. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J Am Geriatr Soc. 2022;70(9):2695-709 https://www.ncbi.nlm.nih.gov/pubmed/36073186.
- 42.Lin CS. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr. 2018;18(1):5 https://www.ncbi.nlm.nih.gov/pubmed/29304748.
- 43.Wu YT, et al. The changing prevalence and incidence of dementia over time – current evidence. Nat Rev Neurol. 2017;13(6):327-39 https://www.ncbi.nlm.nih.gov/pubmed/28497805.
- 44.National Psoriasis Foundation. Soriatane (Acitretin). https://www.psoriasis.org/soriatane-acitretin/.
- 45.National Psoriasis Foundation. Current Biologics on the Market. https://www.psoriasis.org/current-biologics-on-the-market/.
- 46.Dalmády S, Kemény L, Antal M, Gyulai R. Periodontitis: a newly identified comorbidity in psoriasis and psoriatic arthritis. Expert Rev Clin Immunol 2020;16(1):101-8. doi: 10.1080/1744666X.2019.1700113.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2020/06/pre-procedural-rinsing-2.jpg)