Update on Antimicrobial Resistance and the COVID-19 Pandemic
Early in the COVID-19 pandemic, concern was raised about potential inappropriate use of antimicrobials and whether this would impact efforts against antimicrobial resistance (AMR) and antimicrobial stewardship programs (ASP) during the crisis. In this article, we will review the status of AMR pre-pandemic, changes during the pandemic, and current developments.
Pre-pandemic
In one review of bacterial AMR, estimated global deaths in 2019 for 23 pathogens reached 4.95 million, with 1.27 million of these directly attributed to AMR.1Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399(10325):629-55. doi: 10.1016/S0140-6736(21)02724-0. Estimated attributable and associated disability-adjusted life-years (DALYs) were 47.9 million and 192 million, respectively. In the US, more than 3.1 million infections associated with bacterial and fungal AMR occurred in 2019, and 35,900 deaths in hospitalized patients.2CDC. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Globally, six bacterial pathogens were responsible for a majority of deaths related to drug-resistant bacterial infections.1Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399(10325):629-55. doi: 10.1016/S0140-6736(21)02724-0. In the US, drug-resistant Streptococcus pneumoniae, Neisseria gonorrhoeae, Campylobacter, methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile (C. diff) caused the most infections in hospitalized patients.2CDC. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. (Figure 1)
Numbers alone underestimate the scope of the problem. An increasing number of microorganisms are multi-drug resistant, including Klebsiella pneumoniae, Mycobacterium tuberculosis, MRSA, vancomycin-resistant Enterococcus (VRE), C. diff, extended-spectrum beta-lactamase (ESBL) and carbapenem-resistant Enterobacteriaceae, carbapenem-resistant acinetobacter species, Pseudomonas aeruginosa, Neisseria gonorrhoeae and Candida auris.2CDC. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.,3van Duin D, Paterson DL. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect Dis Clin North Am 2016;30(2):377-90. doi: 10.1016/j.idc.2016.02.004. Sixteen microorganisms are now classified as urgent or serious threats, including other drug-resistant microorganisms in addition to those described above.2CDC. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
Antibiotic use in patients with COVID-19
Antibiotic use soared in hospital settings during the pandemic. In a prospective multicenter study across 260 hospitals in Great Britain on antibiotic use between early February and early June, 2020, 85% of hospitalized patients (almost 49,000) with confirmed/suspected COVID-19 were prescribed antimicrobials during admission.4Russell CD, Fairfield CJ, Drake TM et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2021;2(8):e354-65. doi: 10.1016/S2666-5247(21)00090-2. In a retrospective review of US electronic health data for almost 5000 hospitalized patients it was found that more than half received antibiotics, 86% of them immediately and 96% within 48 hours (i.e., before confirmation of any bacterial co-infection).5PEW. Could Efforts to Fight the Coronavirus Lead to Overuse of Antibiotics? Study shows more than half of hospitalized COVID-19 patients in U.S. received antibiotics in pandemic’s first six months. March 10, 2021. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/03/could-efforts-to-fight-the-coronavirus-lead-to-overuse-of-antibiotics. Community-acquired bacterial pulmonary co-infection was later confirmed for 19% of patients (and urinary tract infections for 9% of patients). In a retrospective case study in New York, among the first 1,000 patients with COVID-19, 94.9% received antibiotics.6Argenziano MG, Bruce SL, Slater CL et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. Br Med J 2020;369:m1996. https://doi.org/10.1136/bmj.m1996.
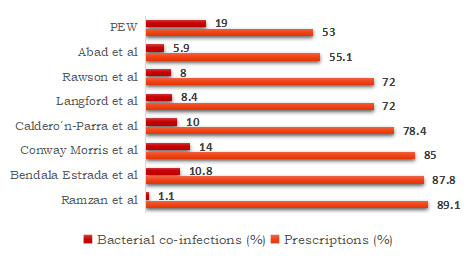
Data from the SEMI-COVID19 Registry entered between March 1 and May 23, 2020, showed that 87.8% of 13,736 hospitalized patients received antibiotics.7Bendala Estrada AD, Calderón Parra J, Fernández Carracedo E et al. Inadequate use of antibiotics in the covid-19 era: effectiveness of antibiotic therapy. BMC Infect Dis 2021;21:1144. https://doi.org/10.1186/s12879-021-06821-1. Among these patients, bacterial pneumonia was reported for 10.8%. In a subsequent report (end date June 23, 2020), 78.4% of patients had received antibiotics, while 10% had pulmonary bacterial coinfection (and 2% non-pulmonary coinfection).8Caldero´n-Parra J, Muiño-Miguez A, Bendala-Estrada AD et al. Inappropriate antibiotic use in the COVID-19 era: Factors associated with inappropriate prescribing and secondary complications. Analysis of the registry SEMI-COVID. PLoS ONE 2021;16(5): e0251340. https://doi.org/10.1371/journal.pone.0251340. A meta-analysis of 24 studies yielded confirmed bacterial infection in 8.4% of patients while almost 72% received antibiotics.9Langford BJ, So M, Raybardhan S et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020. https://doi.org/10.1016/j.cmi.2020.07.016. In December 2020, 9 studies on patients with COVID-19 were included in a review of bacterial/fungal coinfection in patients with infections caused by coronaviruses.10Rawson TM, Moore LSP, Zhu N et al. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin Infect Dis 2020;71(9):2459-68. doi: 10.1093/cid/ciaa530. Among patients with COVID-19, 72% had received antimicrobial therapy, while 8% had a coinfection.
Similar patterns were found in other studies. In a multicenter multi-country European study, 85% of patients received antibiotics when admitted to intensive care units, while bacterial co-infection was found in 14%.11Conway Morris A, Kohler K et al. Co-infection and ICU-acquired infection in COIVD-19 ICU patients: a secondary analysis of the UNITE-COVID data set. Crit Care 2022;26(1):236. doi: 10.1186/s13054-022-04108-8. In a study in the Philippines conducted March through August 2020, 55.1% of patients received antibiotics while 5.9% presented with co-infection.12Abad CL, Sandejas JCM, Poblete JB et al. Bacterial coinfection and antimicrobial use among patients with COVID-19 infection in a referral center in the Philippines: A retrospective cohort study. IJID Reg 2022;4:123-30. doi: 10.1016/j.ijregi.2022.07.003. In a review of patients admitted in Pakistan (up to the last peak in January 2022), 89.7% of patients received antibiotics.13Ramzan K, Shafiq S, Raees I et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics (Basel) 2022;11(6):789. doi: 10.3390/antibiotics11060789. Bacterial co-infections and secondary infections were found in just 1.14% and 3.14%, respectively. (Figure 2) Furthermore, a retrospective review of hospitalized patients with COVID-19 in two London hospitals revealed low levels of bacterial coinfection with rates comparable to a control group with influenza in the prior year.14Hughes S, Troise O, Donaldson H et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020;26(10):1395-9. doi: 10.1016/j.cmi.2020.06.025. Due to insufficient data, it has not been possible to determine rates of prescribing of anti-fungals.
Antibiotic prescribing in the dental setting
Antibiotic prescribing in the dental setting generally increased after onset of the pandemic, driven by practice closures and constraints. In England, antibiotic prescriptions were 25% higher overall for April through June 2020 compared to the same period in 2019.15Shah S, Wordley V, Thompson W. How did COVID-19 impact on dental antibiotic prescribing across England? Br Dent J 2020;229:601-4. https://doi.org/10.1038/s41415-020-2336-6. In Scotland, antibiotic prescriptions were 49% higher after dental care was halted and still 28% higher compared to pre-pandemic after care resumed.16Duncan EM, Goulao B, Clarkson J et al. ‘You had to do something’: prescribing antibiotics in Scotland during the COVID-19 pandemic restrictions and remobilisation. Br Dent J 2021:1-6. doi: 10.1038/s41415-021-3621-8. In public health dental clinics in Alberta, Canada, 76% more antibiotic prescriptions were provided from mid-March through the end of 2020 compared to 2019.17Rabie H, Figueiredo R. Provision of dental care by public health dental clinics during the COVID-19 pandemic in Alberta, Canada. Prim Dent J 2021;10(3):47-54. doi: 10.1177/20501684211029423. Increases were highest for April and May when dental offices were restricted. In Australia, antibiotic prescriptions initially decreased by 18% in April 2020, compared to the same period in 2019.18Mian M, Teoh L, Hopcraft M. Trends in Dental Medication Prescribing in Australia during the COVID-19 Pandemic. JDR Clin Trans Res 2021;6(2):145-52. doi: 10.1177/2380084420986766. In June 2020, a 20% increase in amoxicillin prescriptions was found compared to June 2019. In France, antibiotic prescriptions increased by 17.9% for dentists in 2020 compared to 2019.19Bara W, Brun-Buisson C, Coignard B, Watier L. Outpatient Antibiotic Prescriptions in France: Patients and Providers Characteristics and Impact of the COVID-19 Pandemic. Antibiotics (Basel) 2022;11(5):643. doi: 10.3390/antibiotics11050643. In contrast, they decreased by 30.4% and 17.0% for pediatricians and general practitioners, respectively.
Increases in the dental setting do not include antibiotic prescriptions written in outpatient emergency settings for dental infections when dental clinics were closed or curtailed.
Have changes in levels of antimicrobial resistance occurred?
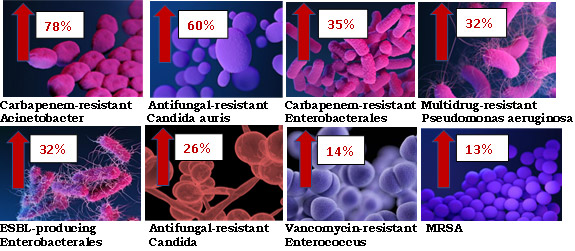
In short, yes. A rapid increase for several multi-drug resistant microorganisms has occurred, most of which fall under the CDC list of ‘Urgent and Serious Threats’.20Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents 2021;57(4):106324. doi: 10.1016/j.ijantimicag.2021.106324.,21CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. https://www.cdc.gov/drugresistance/covid19.html. They include carbapenem-resistant New Delhi metallo-β-lactamase-producing Enterobacterales, ESBL-producing Klebsiella pneumoniae, Acinetobacter baumannii, MRSA and two fungi, including multi-triazole-resistant Aspergillus fumigatus.20Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents 2021;57(4):106324. doi: 10.1016/j.ijantimicag.2021.106324. In the US, increases in hospital-acquired infections have ranged from 13% to 78% for 8 drug-resistant microorganisms.21CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. https://www.cdc.gov/drugresistance/covid19.html. (Figure 3)
Challenges during COVID-19
As we know, dental offices closed shortly after the pandemic began and once re-opened triaging of patients became standard practice. Depending on location, dentists were instructed to manage patients remotely and use antibiotics where appropriate and similar guidance was provided based on location for deferred dental emergencies.15Shah S, Wordley V, Thompson W. How did COVID-19 impact on dental antibiotic prescribing across England? Br Dent J 2020;229:601-4. https://doi.org/10.1038/s41415-020-2336-6.,22Luzzi V, Ierardo G, Bossù M, Polimeni A. COVID-19: Pediatric Oral Health during and after the Pandemics. Appl. Sci 2020;10:1-8. doi: 10.20944/preprints202004.0002.v1. Dentists were concerned that antibiotic use would now increase when they had intended to do the opposite before the pandemic occurred.16Duncan EM, Goulao B, Clarkson J et al. ‘You had to do something’: prescribing antibiotics in Scotland during the COVID-19 pandemic restrictions and remobilisation. Br Dent J 2021:1-6. doi: 10.1038/s41415-021-3621-8. Prior to the pandemic progress was made on antibiotic stewardship. In one example, a successful ASP within academic dental clinics resulted in a 72.9% reduction in antibiotic prescribing.23Gross AE, Hanna D, Rowan SA et al. Successful Implementation of an Antibiotic Stewardship Program in an Academic Dental Practice. Open Forum Inf Dis 2019;6(3):ofz067. https://doi.org/10.1093/ofid/ofz067.
In hospital settings, antimicrobials were frequently prescribed against a background of crisis and uncertainty amid sparse knowledge on COVID-19, an absence of effective therapies and lack of treatment guidelines.7Bendala Estrada AD, Calderón Parra J, Fernández Carracedo E et al. Inadequate use of antibiotics in the covid-19 era: effectiveness of antibiotic therapy. BMC Infect Dis 2021;21:1144. https://doi.org/10.1186/s12879-021-06821-1.,24Peghin M, Vena A, Graziano E et al. Improving management and antimicrobial stewardship for bacterial and fungal infections in hospitalized patients with COVID-19. Ther Adv Inf Dis January 2022. doi:10.1177/20499361221095732. A further factor may have been the recommendation that antibiotic therapy be initiated for patients with community-acquired pneumonia since at disease onset no definitive test exists to inform whether a case is viral/bacterial.7Bendala Estrada AD, Calderón Parra J, Fernández Carracedo E et al. Inadequate use of antibiotics in the covid-19 era: effectiveness of antibiotic therapy. BMC Infect Dis 2021;21:1144. https://doi.org/10.1186/s12879-021-06821-1.,25Metlay JP, Waterer GW, Long AC et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200(7):e45-67. https://doi.org/10.1164/rccm.201908-1581ST. Adherence to ASP decreased. For example, results for an Italian survey conducted in July 2021 in infectious disease hospital units revealed that half of ASP were reduced, and 7 of 9 had ceased.26Comelli A, Genovese C, Lombardi A et al. What is the impact of SARS-CoV-2 pandemic on antimicrobial stewardship programs (ASPs)? The results of a survey among a regional network of infectious disease centres. Antimicrob Resist Infect Control 2022;11(1):108. doi: 10.1186/s13756-022-01152-5. ASP did restart in units where this was a priority. Regionally, programs were widely discontinued.
However, ASP that rapidly modified protocols during the pandemic could reduce the use and duration of antibiotic therapies.24Peghin M, Vena A, Graziano E et al. Improving management and antimicrobial stewardship for bacterial and fungal infections in hospitalized patients with COVID-19. Ther Adv Inf Dis January 2022. doi:10.1177/20499361221095732. In a Scottish study conducted shortly after national antibiotic recommendations for suspected bacterial respiratory tract infection complicating COVID-19 were introduced, antibiotic prescribing rates for a single day (over a period of 10 days) were compared for patients with and without COVID-19 in critical and non-critical care hospital units in April 2020.27Seaton RA, Gibbons CL, Cooper L et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect 2020;81(6):952-60. doi: 10.1016/j.jinf.2020.09.024. Antibiotic prescribing for patients with COVID-19 was relatively low, and in non-critical care units a high proportion were narrow spectrum antibiotics, with broad-spectrum antibiotics spared. Broad spectrum antibiotics were, however, prescribed proportionately more in critical care settings.27Seaton RA, Gibbons CL, Cooper L et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect 2020;81(6):952-60. doi: 10.1016/j.jinf.2020.09.024. In the US, current difficulties include the lack of data available currently on numerous threatening drug-resistant microorganisms for 2020, due to resource constraints and other limitations.21CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. https://www.cdc.gov/drugresistance/covid19.html.
Pipeline Developments
Current research approaches for antimicrobials include novel methods that affect the microorganisms without killing them and compounds that interfere with bacterial metabolic processes.28Robins-Browne R. Disarming Bacterial Virulence. Biology, Health and Medicine. Aug 23, 2017. https://www.scientia.global/professor-roy-robins-browne-disarming-bacterial-virulence/. For example, research on anti-adhesive molecules that could combat adhesive proteins on the surface of pili/fimbrae that foster bacterial adhesion to the bladder wall for some E. coli strains.29Ribić R, Meštrović T, Neuberg M, Kozina G. Effective anti-adhesives of uropathogenic Escherichia coli. Acta Pharmaceutica 2018;68(1):1-18. https://doi.org/10.2478/acph-2018-0004. FabI inhibitors target an enzymatic catalyst involved in bacterial fatty acid production. ‘Fabimycin’ tackles drug-resistant gram-negative bacteria, and in mice has effectively reduced the level of drug-resistant K. pneumoniae.30Parker EN, Cain BN, Hajian B et al. An Iterative Approach Guides Discovery of the FabI Inhibitor Fabimycin, a Late-Stage Antibiotic Candidate with In Vivo Efficacy against Drug-Resistant Gram-Negative Infections. ACS Cent Sci 2022;8(8):1145-58. https://doi.org/10.1021/acscentsci.2c00598. Teixobactin is produced by soil-dwelling bacteria and kills other bacteria present. In vitro, synthetic teixobactins can kill a wide range of microorganisms and in mice shown to eliminate MRSA.31University of Liverpool. Liverpool scientists develop synthetic antibiotics that could save millions of lives. March 29, 2022. https://www.liverpool.ac.uk/health-and-life-sciences/news-and-events/articles/liverpool-scientists-develop-synthetic-antibiotics-that-could-save-millions-of-lives/. A ‘jumbo phage’ has also been developed that produces an outer protective single-protein coating once it enters the bacterial host, creating a barrier against bacterial attack while the jumbo phage can still release its genetic material.32Laughlin TG, Deep A, Prichard AM et al. Architecture and self-assembly of the jumbo bacteriophage nuclear shell. Nature 2022;608:429-35. https://doi.org/10.1038/s41586-022-05013-4. Closer to home, in newly-published research, a second-generation antimicrobial peptide (DGL13K) derived from a salivary protein has been found in vitro to offer antimicrobial efficacy against several drug-resistant and other bacteria.33Gorr S-U, Brigman HV, Anderson JC, Hirsch EB. The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS ONE 2022;17(8): e0273504. https://doi.org/10.1371/journal.pone.0273504.
Conclusions
While ASP were gaining ground and reductions in prescribing of antimicrobials were being achieved, COVID-19 resulted in increased prescribing and increased AMR and drug-resistant infections. Appropriately adapted ASP have been shown to work. In addition, electronic health records and e-prescribing offer opportunities to better track prior prescriptions and make more informed decisions. There is also promising research on innovative antimicrobials, however, this takes a significant investment. It also takes considerable time, many drug candidates do not come to fruition, and the stock of effective antimicrobials is dwindling. In the meantime, more attention to tracking AMR, further integration of ASP, resources for antimicrobial drug development, vaccinations, increased infection prevention efforts and new technologies are needed to contain threats and prevent drug-resistant outbreaks.
References
- 1.American Association of Pediatric Dentistry. Guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Reference Manual 2013;39(6):412-9.
- 2.Andersson L, Andreasen JO, Day P, Heithersay G, Trope M, DiAngelis AJ, Kenny DJ, Sigurdsson A, Bourguignon C, Flores MT, Hicks ML, Lenzi AR, Malmgren B, Moule AJ, Tsukiboshi M. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol 2012;28:88-96.
- 3.Bagattoni S, Sadotti A, D’Alessandro G, Piana G. Dental trauma in Italian children and adolescents with special health care needs. A cross-sectional retrospective study. Eur J Paediatr Dent 2017;18:23-6.
- 4.Lee JY, Divaris K. Hidden consequences of dental trauma: The social and psychological effects. Pediatr Dent 2009;31:96-101.
- 5.American Association of Endodontists. The recommended guidelines of the American Association of Endodontists for the treatment of traumatic dental injuries, 2013. Available at: http://www.nxtbook.com/nxtbooks/aae/traumaguidelines/index.php#/4
- 6.Petrovic B, Marković D, Peric T, Blagojevic D. Factors related to treatment and outcomes of avulsed teeth. Dent Traumatol 2010;26(1):52-9. doi: 10.1111/j.1600-9657.2009.00836.x.
- 7.Poi WR, Sonoda CK, Martins CM, Melo ME, Pellizzer EP, de Mendonça MR, Panzarini SR. Storage media for avulsed teeth: a literature review. Braz Dental J 2013;24(5):437-45.
- 8.Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endod Dent Traumatol 1995;11(2):76-89.
- 9.Tuna EB, Arai K, Tekkesin MS, Seymen F, Gencay K, Kuboyama N, Maeda T. Effect of fibroblast growth factor and enamel matrix derivative treatment on root resorption after delayed replantation. Dent Traumatol 2015;31(1):49-56. doi: 10.1111/edt.12141.
- 10.Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 2. Factors related to pulpal healing. Endod Dent Traumatol 1995;11(2):59-68.
- 11.Andreasen JO, Borum MK, Andreasen FM. Replantation of 400 avulsed permanent incisors. 3. Factors related to root growth. Endod Dent Traumatol 1995;11(2):69-75.
- 12.Is Khinda V, Kaur G, G SB, Kallar S, Khurana H. Clinical and practical implications of storage media used for tooth avulsion. Int J Clin Pediatr Dent 2017;10:158-65.
- 13.Hasan MR, Takebe H, Shalehin N, Obara N, Saito T, Irie K. Effects of tooth storage media on periodontal ligament preservation. Dent Traumatol 2017;33:383-92.
- 14.Kokkali VV, Bendgude V, Sharangpani G. Comparative evaluation of post-traumatic periodontal ligament cell viability using three storage media. Eur Arch Paediatr Dent 2017;18:209-14.
- 15.Saini D, Gadicherla P, Chandra P, Anandakrishna L. Coconut milk and probiotic milk as storage media to maintain periodontal ligament cell viability: an in vitro study. Dent Traumatol 2017;33(3):160-4.
- 16.Lee W, Stover S, Rasoulianboroujeni M, Sherman K, Fahimipour F. The efficacy of commercial tooth storage media for maintaining the viability of human periodontal ligament fibroblasts. Int Endod J 2018;51:58-68.
- 17.de Souza BD, Bortoluzzi EA, Reyes-Carmona J, Dos Santos LG, Simões CM, Felippe WT, Felippe MC. Effect of temperature and seven storage media on human periodontal ligament fibroblast viability. Dent Traumatol 2017;33(2):100-5. doi: 10.1111/edt.12311.
- 18.Ozer S, Yilmaz E, Bayrak S, Tunc ES. Parental knowledge and attitudes regarding the emergency treatment of avulsed permanent teeth. Eur J Dent 2012;6(4):370-5.
- 19.Tiano L, Barbiero S, Avato FA, Bergmann M, Gaudio RM. Management of facial trauma with tooth avulsion in children and adolescent, investigation on knowledge of parents and teachers. Eur J Pub Health 2015;25(Suppl 3): October 2015. https://doi.org/10.1093/eurpub/ckv176.241
- 20.Phelps JLB. Virginia emergency department physician knowledge of the emergent treatment of avulsed teeth. Thesis, Virginia Commonwealth University, 2008. Available at: https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=1844&context=etd.
- 21.Kotha A, Kumar YV, Prathima V, Pratibha B, Ankitha CH. Evaluation of awareness of first aid of avulsed tooth among physicians and nurses of hospital emergency departments: A short survey. Int J Comm Health Med Res 2017;3(3):74-7.
- 22.Baginska J, Rodakowska E Milewski R, Wilczynska-Borawska M, Kierklo A. Polish school nurses’ knowledge of the first-aid in tooth avulsion of permanent teeth. BMC Oral Health 2016;16:30.
- 23.Abu-Dawoud M, Al-Enezi R, Andersson L. Knowledge of emergency management of avulsed teeth among young physicians and dentist. Dental Traumatol 2008;23(6):348-55.
- 24.Raibrown A, Giblin LJ, Boyd LD, Perry K. Gastroesophageal Reflux Disease Symptom Screening in a Dental Setting. J Dent Hyg 2017;91:44-8.
- 25.Drugs.com H2 antagonists. https://www.drugs.com/drug-class/h2-antagonists.html.
- 26.Drugs.com. Protein pump inhibitors. https://www.drugs.com/drug-class/proton-pump-inhibitors.html
- 27.Drugs.com. Sucralfate. https://www.drugs.com/mtm/sucralfate.html.
- 28.Magalhães AC, Wiegand A, Rios D, Buzalaf MAR, Lussi A. Fluoride in dental erosion. Monogr Oral Sci 2011;22:158-70. doi: 10.1159/000325167.
- 29.Teixeira L, Manarte-Monteiro P, Manso MC. Enamel lesions: Meta-analysis on effect of prophylactic/therapeutic agents in erosive tissue loss. J Dent Sci 2016;11(3):215-24. doi: 10.1016/j.jds.2016.03.008.
- 30.Hines D, Xu S, Stranick M, Lavender S, Pilch S, Zhang YP, et al. Effect of a stannous fluoride toothpaste on dentinal hypersensitivity: In vitro and clinical evaluation. J Am Dent Assoc 2019;150(4S):S47-S59. doi: JADA article
- 31.American Dental Association. Erosive tooth wear. https://www.ada.org/en/member-center/oral-health-topics/erosive-tooth-wear.
- 32.Scully CBE C. Drug effects on salivary glands: dry mouth. Oral Dis 2003;9:165-76. doi:10.1034/j.1601-0825.2003.03967.x
- 33.Tang KD, Vasani S, Taheri T, Walsh LJ, Hughes BGM, Kenny L, Punyadeera C. An occult HPV-driven oropharyngeal squamous cell carcinoma discovered through a saliva test. Front Oncol 2020;10:408. doi: 10.3389/fonc.2020.00408.
- 34.OralDNA. OraRisk HPV. https://www.oraldna.com/test/ohpv-complete/
- 35.Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med 2015;7:293ra104. doi: 10.1126/scitranslmed.aaa8507.
- 36.Zielińska K, Karczmarek-Borowska B, Kwaśniak K, Czarnik-Kwaśniak J, Ludwin A, Lewandowski B, Tabarkiewicz J. Salivary IL-17A, IL-17F, and TNF-α are associated with disease advancement in patients with oral and oropharyngeal cancer. J Immunol Res 2020:3928504. doi: 10.1155/2020/3928504.
- 37.Lee LT, Wong YK, Hsiao HY, Wang YW, Chan MY, Chang KW. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2018;47(6):699-707. doi: 10.1016/j.ijom.2017.09.016.
- 38.Shen S, Saito Y, Ren S, Liu C, Guo T, Qualliotine J, et al. Targeting viral DNA and promoter hypermethylation in salivary rinses for recurrent HPV-positive oropharyngeal cancer. Otolaryngol Head Neck Surg 2020;162(4):512-9. doi: 10.1177/0194599820903031.
- 39.Hanna GJ, Lau CJ, Mahmood U, Supplee JG, Mogili AR, Haddad RI, et al. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol 2019;95:120-6. doi: 10.1016/j.oraloncology.
- 40.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am 2011;55(1):159-78. doi:10.1016/j.cden.2010.08.004.
- 41.Collins FM. Integrating the 4Ps into patient care. Available at: https://www.colgateoralhealthnetwork.com/article/integrating-the-4ps-into-patient-care/.
- 42.Knight ET, Murray Thomson W. A public health perspective on personalized periodontics. Periodontol 2000. 2018 Oct;78(1):195-200. doi: 10.1111/prd.12228. PMID: 30198135.
- 43.Picciani BLS, Domingos TA, Teixeira-Souza T et al. Evaluation of the Th17 pathway in psoriasis and geographic tongue. An Bras Dermatol 2019;94(6):677-83. doi: 10.1016/j.abd.2019.01.006.
- 44.National Psoriasis Foundation. Soriatane (Acitretin). https://www.psoriasis.org/soriatane-acitretin/.
- 45.National Psoriasis Foundation. Current Biologics on the Market. https://www.psoriasis.org/current-biologics-on-the-market/.
- 46.Dalmády S, Kemény L, Antal M, Gyulai R. Periodontitis: a newly identified comorbidity in psoriasis and psoriatic arthritis. Expert Rev Clin Immunol 2020;16(1):101-8. doi: 10.1080/1744666X.2019.1700113.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2022/09/update-on-antimicrobial-resistance-2.jpg)